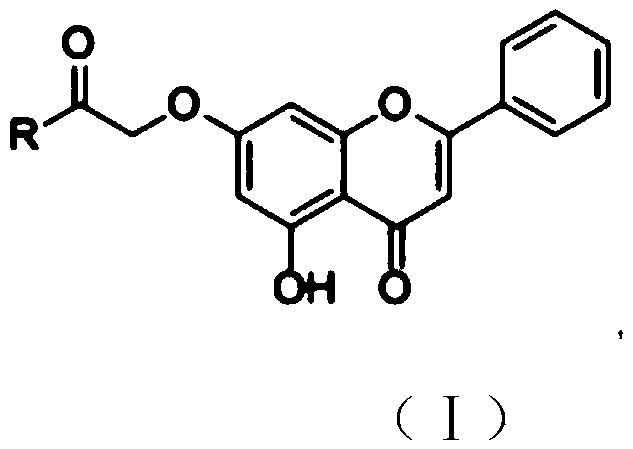
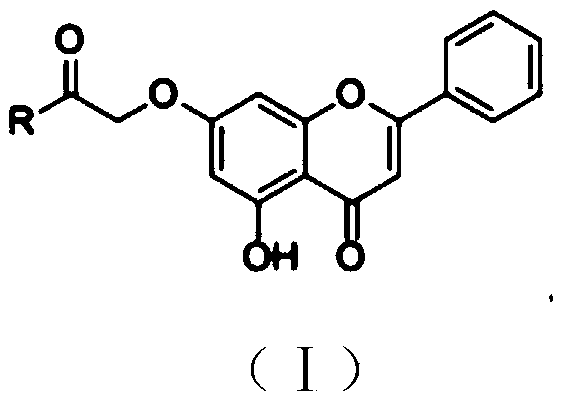
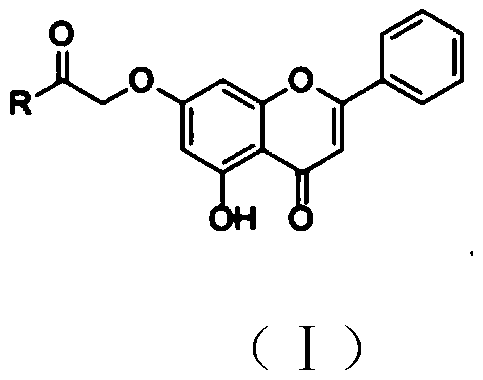
Chrysin amide derivative as well as preparation method and medical application thereof
A technology of coyne amide and its derivatives, applied in the field of coyne amide derivatives and their preparation, can solve the problems that there are no research reports on uric acid-lowering and anti-gout of coyne amide derivatives, and achieve the effect of good water solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Embodiment 1: Preparation of coyne amide derivatives
[0028] 1. Add coyne and anhydrous potassium carbonate to an appropriate amount of acetone and stir, then add ethyl bromoacetate to the solution, and heat and reflux under the condition of stirring while heating; wherein coyne, anhydrous potassium carbonate , The molar ratio of ethyl bromoacetate is 1:(1~2):(1~2). After the reaction was over, it was cooled. Suction filter the solution, concentrate the filtrate to a density of 1.1-1.3 after suction filtration, wash the filtrate concentrate with petroleum ether, 1wt% NaOH solution and pure water successively, then vacuum-dry and purify through a silica gel column.
[0029]
[0030] 2. Dissolve the product obtained in step 1 in an appropriate amount of methanol, add KOH to the solution and reflux under heating and stirring to dissolve; wherein the molar ratio of KOH to coine is (0.2-0.5):1. Suction filtration, the filtered solid was washed with methanol and dried. ...
Embodiment 2
[0035] Example 2: Pharmacological Activity Screening of Coinamide Derivatives
[0036] 1. Screening for inhibition of xanthine oxidase activity
[0037] The biochemical basis of hyperuricemia and gout is excessive uric acid concentration in body fluids, and reducing excessive uric acid is the main treatment strategy. Xanthine oxidase (Xanthine oxidase, XO) is a key enzyme in the production of uric acid, which catalyzes the production of uric acid from hypoxanthine and xanthine, and at the same time produces a large amount of active oxygen such as superoxide anion and hydrogen peroxide. Therefore, XO is a good high Targets for treatment of uric acidemia and gout.
[0038] 1.1 Preparation of reagents
[0039] 1.1.1 Preparation of test solution
[0040] (1) Preparation of dipotassium hydrogen phosphate buffer solution (pH=7.5, 50mM, prepared according to the appendix of "Pharmacopoeia of the People's Republic of China" 2010 edition): that is, accurately weigh the anhydrous hyd...
Embodiment 3
[0064] Embodiment 3: oil-water partition coefficient
[0065] The oil-water partition coefficient of each compound was determined by the classic n-octanol-water system, wherein the oil-water partition coefficient IgP of coyne was 4.52, and the oil-water partition coefficient IgP of other coyne amide derivatives is shown in the sixth column of Table 1. It can be seen from the table that the oil-water partition coefficients of coyne amide derivatives prepared in Example 1 are better than those of coyne, indicating that they have better water solubility and better druggability.
[0066] Table 1. IC50 values and IgP of coyne series derivatives inhibiting xanthine oxidase, TNF-α and IL-1β
[0067]
[0068]
[0069]
[0070]
[0071]
[0072]
[0073]
[0074]
[0075]
[0076]
[0077]
[0078]
[0079] Conclusion: The coyne amide derivatives proposed in the present invention have good water solubility, stability and pharmacological activity ch...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com