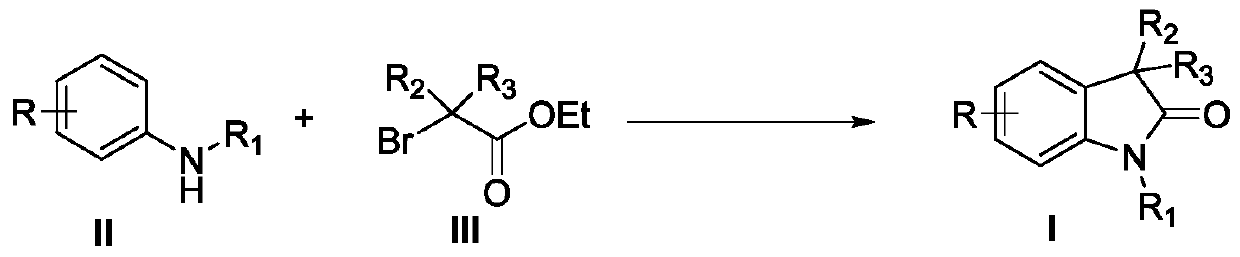
Synthesis method of 3,3'-disubstituted-2-indolone compound
A synthesis method and compound technology, applied in 3 fields, can solve the problems of complex synthesis process steps and high cost, and achieve the effects of wide adaptability, low price and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-20
[0030] Embodiment 1-20 reaction condition optimization test
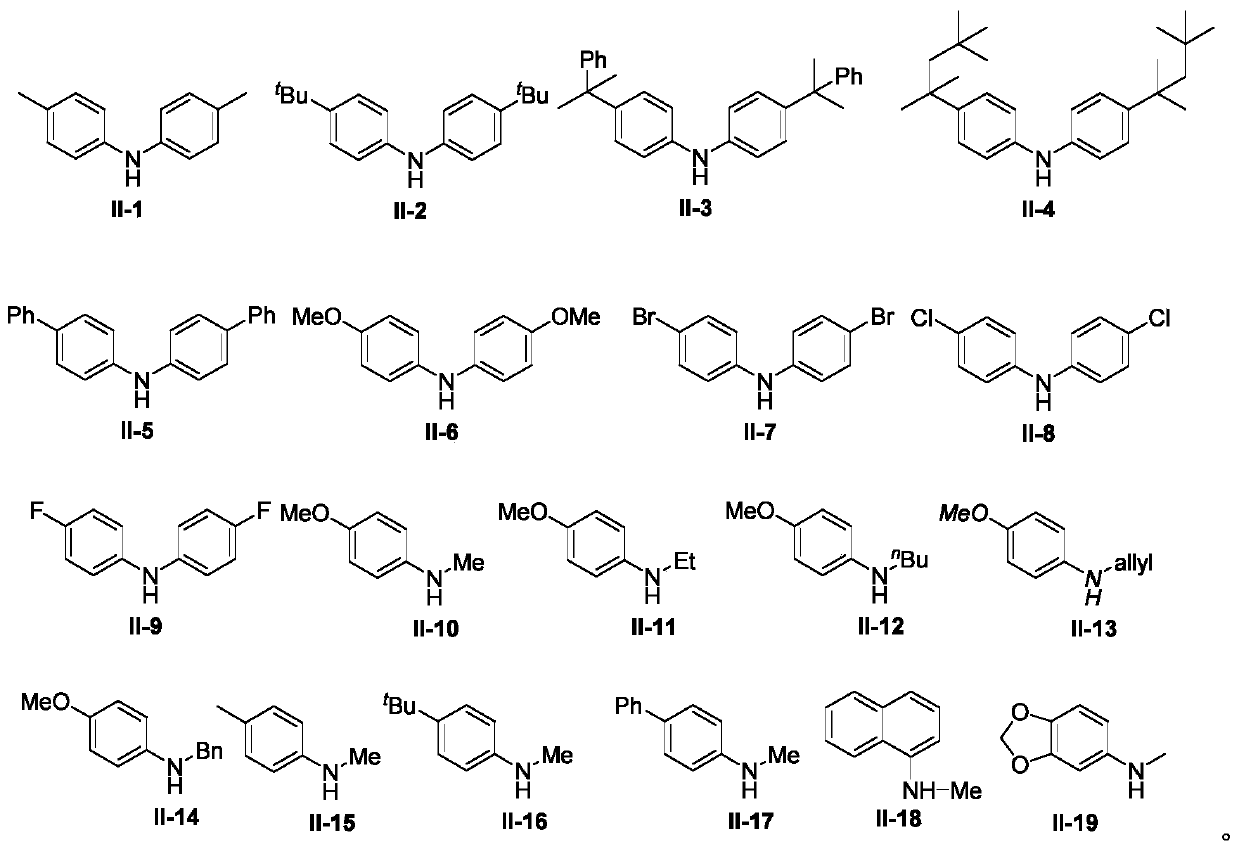
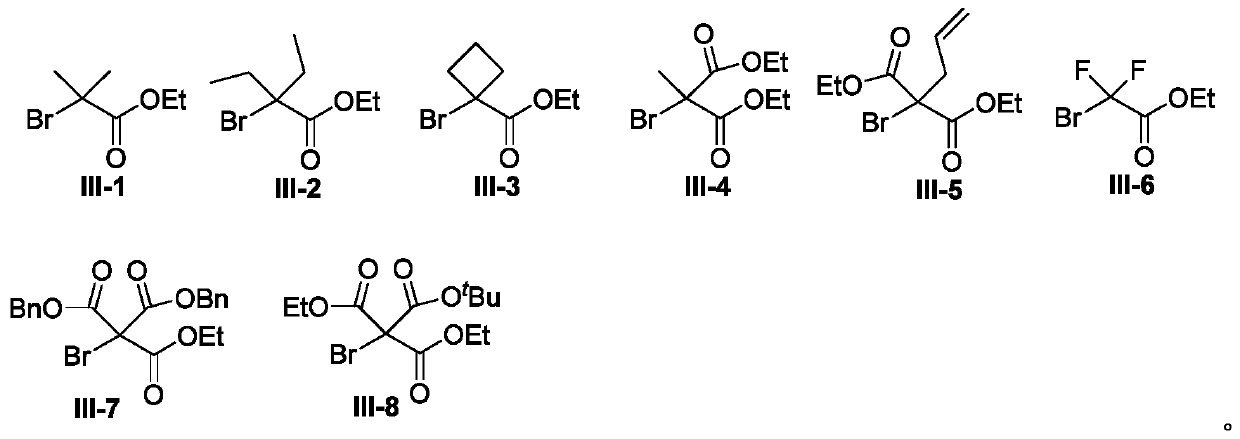
[0031] With di-p-tolylamine shown in formula II-1 and 2-bromo-2-methylpropionate raw materials shown in formula III-1, the influence of different reaction conditions on the process optimization results was discussed, and the Representative examples 1-20, the results are shown in Table 1:
[0032]
[0033] Wherein, the test operation of embodiment 1 is as follows:
[0034] In the dry, airtight 25mL Schlenk sealed tube reactor, add the xylamine (0.3mmol) shown in the formula II-1, the 2-bromo-2-methylpropionic acid ethyl alcohol shown in the formula III-1 successively Esters (2equiv, 0.6mmol), Cu(OAc) 2 (10mol%), 2,2'-bipyridine (20mol%), K 2 CO 3 (2equiv, 0.6mmol), followed by adding the solvent acetonitrile (2mL), and reacting at 120°C for 12h. After the reaction was complete, the reaction solution was concentrated in vacuo, and the residue was separated by silica gel column chromatography (n-hexane / ethyl ac...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com