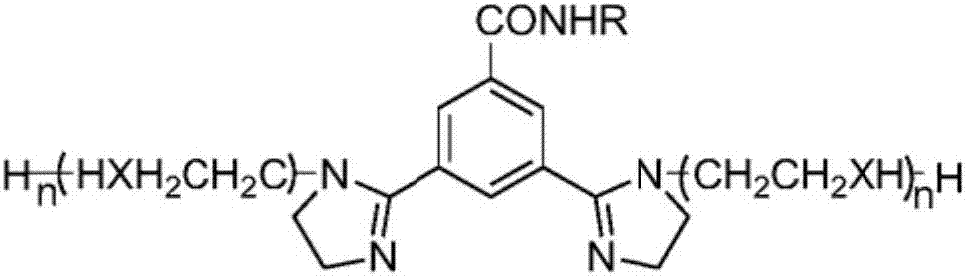
Benzotriazole imidazoline derivative, and preparation method and application thereof
A technology of benzotriazole imidazoline and benzotriazole, which is applied in the field of benzotriazole derivatives and its synthesis process, can solve the problems of low water viscosity, difficulty in forming a fluid lubricating film, extreme pressure and anti-wear It has the advantages of simple preparation process, easy operation and preparation, and good corrosion inhibition performance.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
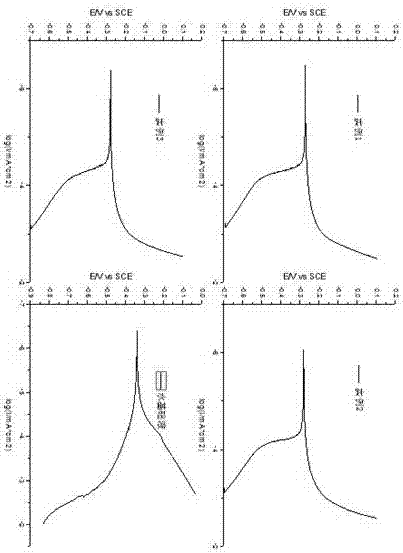
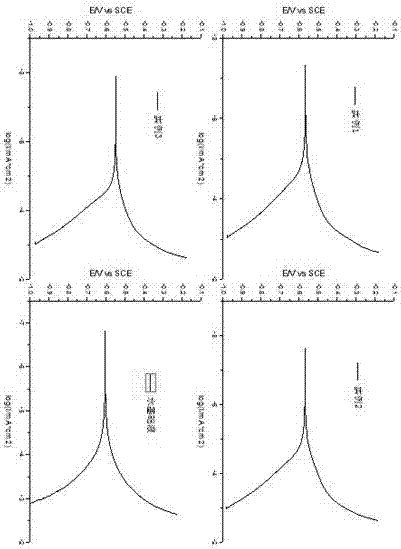
Image
Examples
Embodiment 1
[0047] Example 1: Add 11.90g of benzotriazole to a 250ml three-necked flask, add 100ml of water as a solvent, add 5g of sodium hydroxide, stir at 90°C for 3 hours, add 11.65g of sodium chloroacetate, and react at 90°C After 3 hours, the product was cooled at room temperature and HCl was added until a large amount of precipitation, filtered and washed with water to remove chloride ions, and dried to obtain benzotriazole acetic acid, wherein the yield of sodium chloroacetate was as high as about 50%. Take 17.8 g of benzotriazole acetic acid, the product of the previous step, and add it to a three-necked flask, then add 12.38 g of diethylenetriamine, and add 50 ml of xylene as a solvent and a water-carrying agent. React at 140-160°C for 4 hours to remove the water generated in the reaction, react at 190-210°C for 2 hours to remove the water generated in the reaction, and then distill under reduced pressure for 1 hour to remove unreacted raw materials, that is, brown viscous liquid...
Embodiment 2
[0048] Example 2: Add 11.90g of benzotriazole to a 250ml three-necked flask, add 100ml of water as a solvent, add 5g of sodium hydroxide, stir at 92°C for 3.2 hours, add 11.65g of sodium chloroacetate, and react at 92°C After 3.2 hours, the product was cooled at room temperature and HCl was added until a large amount of precipitation, filtered and washed with water to remove chloride ions, and dried to obtain benzotriazole acetic acid, wherein the yield of sodium chloroacetate was about 50% higher. Take 17.8 g of benzotriazole acetic acid, the product of the previous step, and add it to a three-necked flask, then add 25.07 g of triethylenetetramine, and add 50 ml of xylene as a solvent and a water-carrying agent. React at 140-160°C for 6 hours to remove the water generated in the reaction, react at 190-210°C for 3 hours to remove the water generated in the reaction, and then distill under reduced pressure for 1 hour to remove unreacted raw materials, that is, brown viscous liqu...
Embodiment 3
[0049] Example 3: Add 11.90g of benzotriazole to a 250ml three-necked flask, add 100ml of water as a solvent, add 5g of sodium hydroxide, stir at 88°C for 2.8 hours, add 11.65g of sodium chloroacetate, and react at 88°C After 2.8 hours, the product was cooled at room temperature and HCl was added until a large amount of precipitation, filtered and washed with water to remove chloride ions, and dried to obtain benzotriazole acetic acid, wherein the yield of sodium chloroacetate was about 50% higher. Take 17.8 g of benzotriazole acetic acid, the product of the previous step, and add it to a three-necked flask, then add 22.72 g of tetraethylenepentamine, and add 50 ml of xylene as a solvent and a water-carrying agent. React at 140-160°C for 5 hours to remove the water generated in the reaction, react at 190-210°C for 2 hours to remove the water generated in the reaction, and then distill under reduced pressure for 1 hour to remove unreacted raw materials, that is, brown viscous li...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com