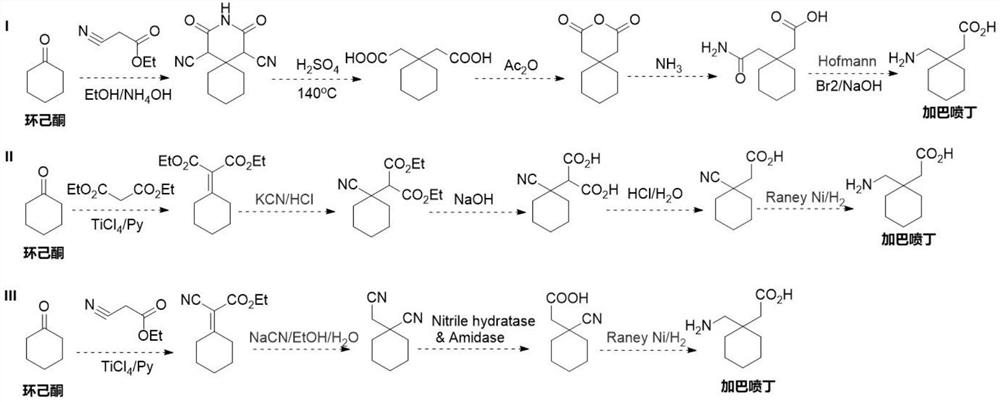
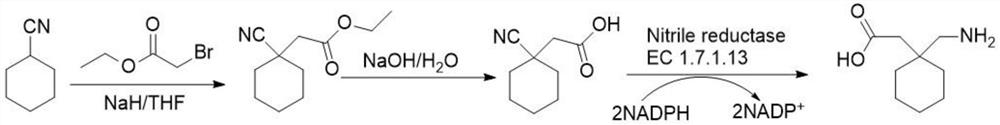
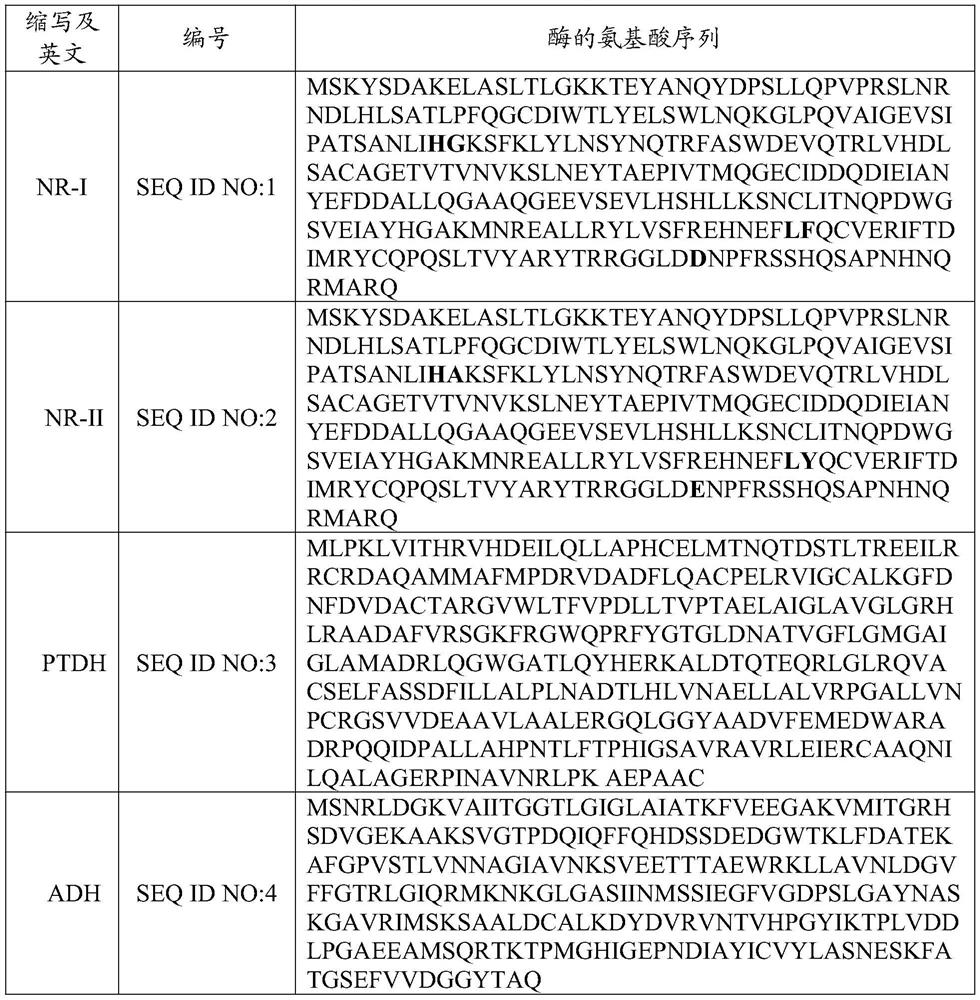
Preparation method of cyano reductase and gabapentin
A gabapentin and reductase technology, applied in biochemical equipment and methods, enzymes, enzymes, etc., can solve the problems of high risk factor, expensive, low efficiency, etc., achieve mild reaction conditions, increase overall yield, and reduce reaction steps Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0054] Preparation of related enzymes:
[0055] The genes required for the above enzymes were directly synthesized by the company (Anhui General Biology), and then subcloned into the pET28a plasmid with NdeI / XhoI restriction sites. The constructed plasmid was transferred into E.coli (BL21) strain (General Biology), and after plate culture, a single clone was picked and transferred to 5 mL of LB medium (37°C) containing 50 μM kanamycin for culture. When the cells grew to the right After several phases (OD ~ 0.6), 0.5 mM isopropyl-β-D-thiogalactopyranoside (IPTG) was added to induce protein expression for 4 hours, and finally the supernatant was obtained by cell collection, disruption and high-speed centrifugation, and then Use sodium dodecylsulfonate-polyacrylamide gel electrophoresis (SDS-PAGE) to confirm that the protein expression is correct, and then the bacteria can be gradually inserted into a 5L culture fermenter for growth at 37°C and induced expression with 0.5mM IPTG ...
Embodiment 1
[0058] Embodiment 1: Synthesis of 2-cyano-2-cyclohexane ethyl acetate
[0059]
[0060] First, cyclohexylcyanide (5.45g, 50mmol) and anhydrous tetrahydrofuran (200mL) were mixed into a three-necked round-bottomed flask, cooled to -10°C in an ice-salt bath, and then solid sodium hydride (1.44g, 62mmol) was added in batches to maintain After the mixture was stirred at low temperature (-10°C) for 10 minutes, ethyl 2-bromoacetate (9.7 mL, 75 mmol) was slowly added dropwise. Stir at low temperature for 15 minutes, then rise to room temperature and stir for 60 minutes, add ice water (200 mL) to terminate the reaction, extract the product with ethyl acetate, dry over sodium sulfate, concentrate and remove the solvent to obtain a solid crude product, and the final product is in ethyl acetate / ethanol=1: 3 (volume ratio) crystallized to obtain 7.2 g of white solid, yield 74%.
Embodiment 2
[0061] Embodiment 2: the synthesis of 2-cyano group-2-cyclohexyl acetic acid
[0062]
[0063] Add sodium hydroxide (2.0g, 50mmol) to the mixed solvent of ionized water (200mL) and ethanol (50mL) to dissolve completely, then add ethyl 2-cyano-2-cyclohexyl acetate (5.86g, 30mmol), The mixed solution was heated and stirred at 50°C for 2 hours, then cooled to room temperature, and finally extracted with ethyl acetate, dried, and concentrated to obtain 5.4 g of crude product, which was directly used in the next step without purification.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com