Sofalcone preparation method
A technology of sofalone and dihydroxyacetophenone, which is applied in the field of preparation of sofalone, can solve the problems of complex process, long reaction steps, low reaction yield and total yield, and achieve shortening of reaction steps and manufacturing cost Reduced effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
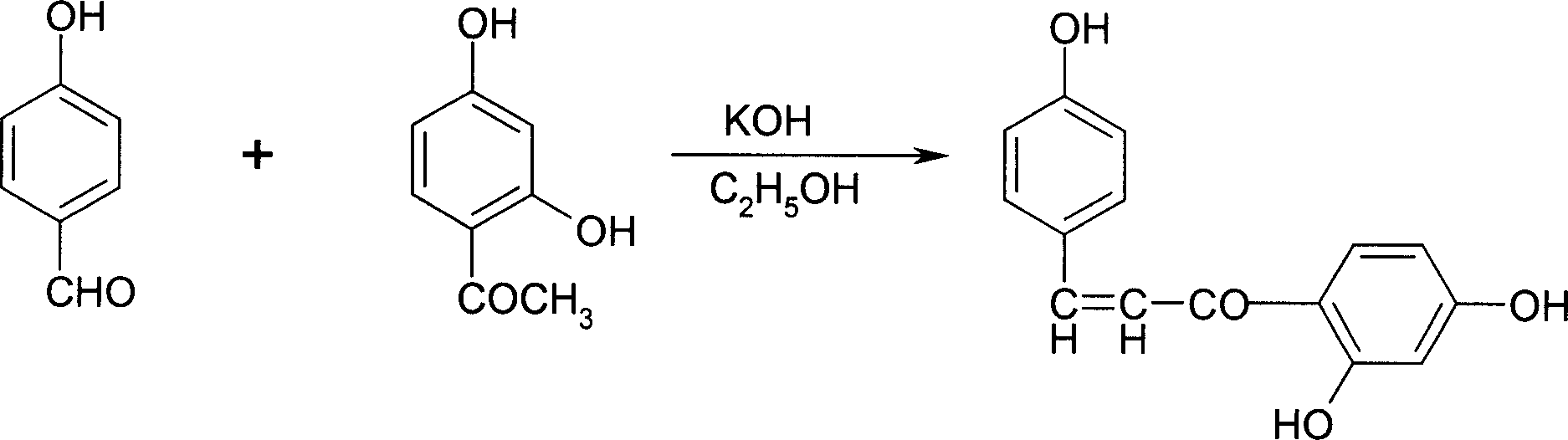
[0029] 1. Add 122 grams (1.00mol) of p-hydroxybenzaldehyde and 152 grams (1.00mol) of 2,4-dihydroxyacetophenone into a 5000mL three-necked bottle, start stirring, add 1500ml of ethanol, cool to 5°C, drop 1150 ml of 20% potassium hydroxide aqueous solution was added, and the stirring reaction was continued for 24 hours. After the reaction was completed, the reactant was poured into ice water, and 16% hydrochloric acid was added dropwise with stirring, neutralized to neutral, continued to stir for 1 hour, filtered, washed with water until neutral, dried, and recrystallized with ethanol to obtain 213 grams of the product, the yield : 83.2%, melting point 201-202.5°C.
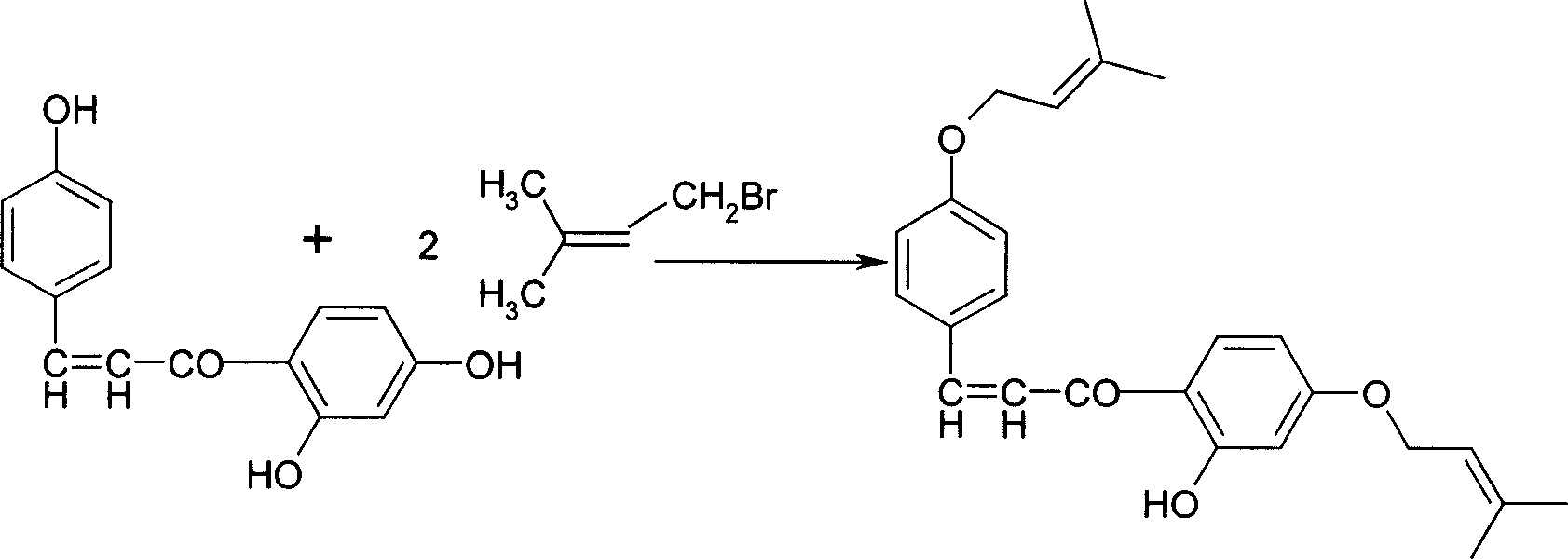
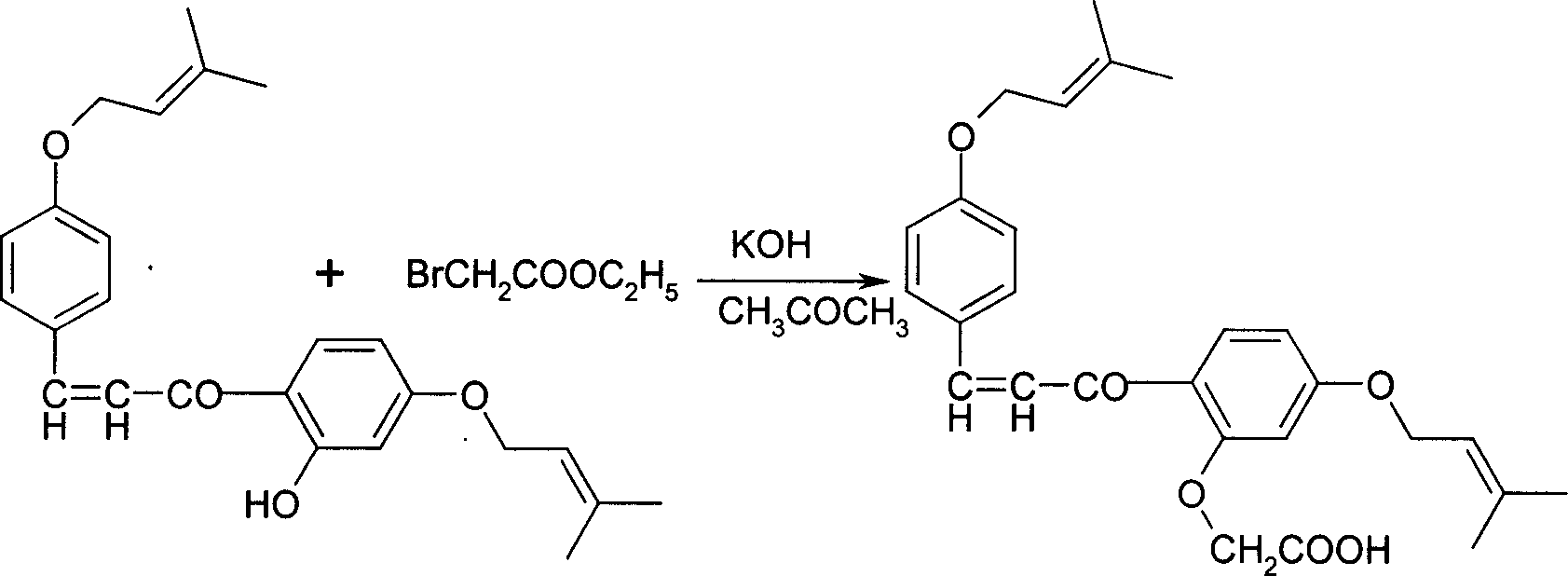
[0030] 2. In a 3000ml three-necked bottle, add 210g (0.82mol) 2,4,4-trihydroxychalcone and 1500ml acetone, start stirring, add 340g (2.46mol) potassium carbonate, stir to dissolve all . Start to add 244.4 grams (1.64mol) of bromoisoamylene dropwise, drop it in 20 minutes, start to heat up to reflux, keep the refl...
Embodiment 2
[0033] 1. Add 122 grams (1.00mol) of p-hydroxybenzaldehyde and 136.8 grams (0.90mol) of 2,4-dihydroxyacetophenone into a 5000mL three-necked flask, start stirring, add 1500ml of ethanol, and cool down to -5°C. 1150 ml of 20% potassium hydroxide aqueous solution was added dropwise, and the stirring reaction was continued for 30 hours. After the reaction was completed, the reactant was poured into ice water, and 16% hydrochloric acid was added dropwise with stirring, neutralized to neutral, and stirred for 1 hour, filtered, washed with water until neutral, dried, and recrystallized with ethanol to obtain 207 grams of the product, yield : 80.9%, melting point 201-202.5°C.
[0034] 2. In a 3000ml three-necked bottle, add 210g (0.82mol) 2,4,4-trihydroxychalcone and 1500ml acetone, start stirring, add 340g (2.46mol) potassium carbonate, stir to dissolve all . Start to add 244.4 grams (1.40 mol) of bromoisoamylene dropwise, finish dropping in 20 minutes, start to heat up to 40°C, k...
Embodiment 3
[0037] 1. Add 122 grams (1.00mol) of p-hydroxybenzaldehyde and 197.6 grams (1.30mol) of 2,4-dihydroxyacetophenone into a 5000mL three-necked flask, start stirring, add 1500ml of ethanol, cool to 15°C, drop 1150 milliliters of 20% potassium hydroxide aqueous solution was added, and the stirring reaction was continued for 36 hours. After the reaction was completed, the reactant was poured into ice water, and 16% hydrochloric acid was added dropwise with stirring, neutralized to neutral, and stirred for 1 hour, filtered, washed with water until neutral, dried, and recrystallized with ethanol to obtain 216 grams of the product, the yield : 84.4%, melting point 201-202.5°C.
[0038] 2. In a 3000ml three-necked bottle, add 210g (0.82mol) 2,4,4-trihydroxychalcone and 1500ml acetone, start stirring, add 340g (2.46mol) potassium carbonate, stir to dissolve all . Start to add 308.5 grams (2.07 mol) of bromoisoamylene dropwise, finish dropping in 20 minutes, stir and react at room temp...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com