Alpha-glycosidase inhibitor preparation method and purpose
A technology of glucosidase and inhibitory activity, which can be used in pharmaceutical formulations, medical preparations containing active ingredients, metabolic diseases, etc., and can solve problems such as high economic pressure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
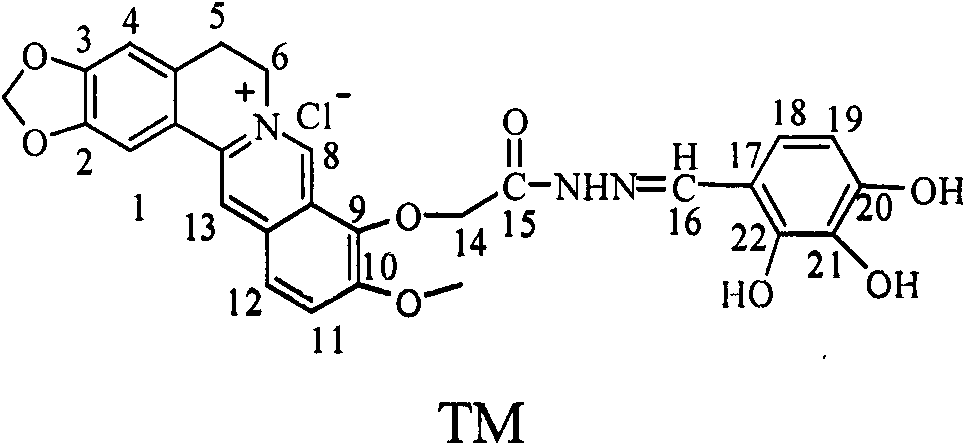
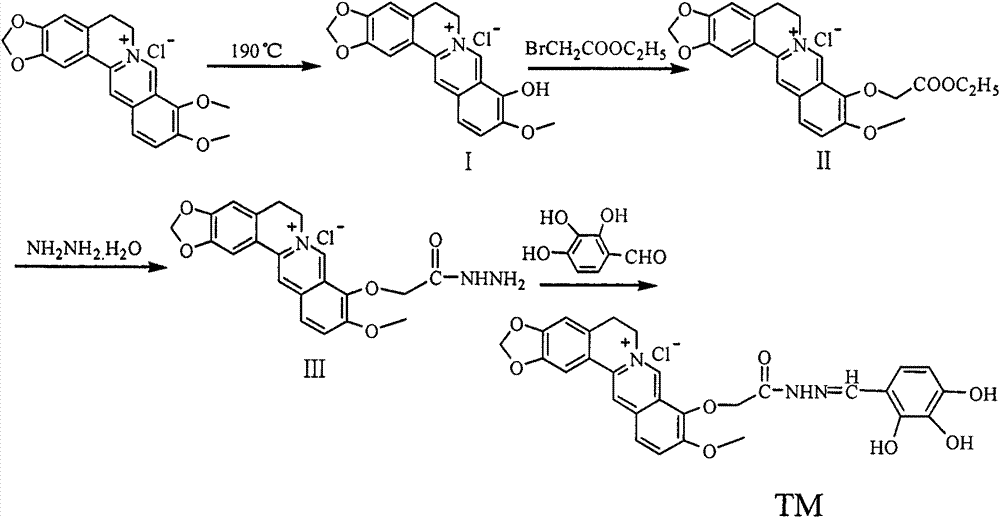
[0023] Embodiment one: the synthesis of berberine (I)
[0024] 10 g (50 mmol) of berberine hydrochloride was decompressed to 20-30 mmHg in a vacuum oven, heated to 190° C. and reacted for 2 h to obtain the target product with a yield of 78%. m.p.169-172°C.
Embodiment 2
[0025] Embodiment two: the synthesis of 9'-O-berberine ethyl acetate hybrid (II)
[0026] Take 5.4g (15mmol) of refined berberine, place it in a dry 250mL round bottom flask with a magnet, add 100mL DMF, dissolve it completely, add 3g of anhydrous K 2 CO 3 , stirred at room temperature for 0.5 h, slowly added 3 g (18 mmol) of ethyl bromoacetate dropwise, continued to stir the reaction, and tracked the reaction with TLC. After the reaction is complete, the reaction solution is filtered, the filter cake is washed with a small amount of DMF, and methanol-chloroform is used as the eluent, and the filtrate is separated by column chromatography on silica gel to obtain 2.77 g of a light yellow solid, and the yield is 42%. m.p.189~191℃.
Embodiment 3
[0027] Embodiment three: the synthesis of 9'-O-berberine hydrazide (III)
[0028] 1.54g (3.46mmol) of compound II, 0.24g (4.16mmol) of 85% hydrazine hydrate, and 40mL of ethanol were stirred and refluxed in a 100mL three-necked flask for 2h, followed by TLC until the end of the reaction. Crystallized by cooling, filtered to obtain 1.16g of crude product, recrystallized with absolute ethanol, dried to obtain 125g of white solid, yield 78.0%, m.p.210-212°C.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com