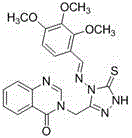
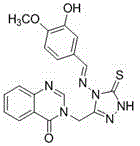
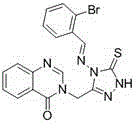
Synthetic method and application of quinazolinone compounds containing 1,2,4-triazolethione Schiff base
A technology of triazolethione Schiff base and quinazolinone is applied in the synthesis and application fields of quinazolinone compounds containing 1,2,4-triazolethione Schiff base, and can solve the problem of high toxicity , low efficacy, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Example 1: Compound 3-((4-(benzylamino)-5-thione-4,5-dihydro-1 H -1,2,4-triazol-3-yl)methyl)quinazoline-4(3 H )-ketone synthesis:
[0043] (1) Preparation of quinazoline-4-one
[0044] Add 9.0g (59.54mmol) methyl anthranilate and 3mL (79.52mmol) formic acid successively into a 100mL three-necked flask, stir well, add 13mL (327.3mmol) formamide, heat up to 130-140°C for reflux reaction, after 6h Stop the reaction, pour the reaction liquid into an appropriate amount of cold water after cooling, a large amount of white solids precipitate, continue to stir for 0.5h, filter with suction, wash with water, dry, and recrystallize with absolute ethanol to obtain 4.10 g of white flocs, with a yield of 47.1 %.
[0045] (2) 2-(4-oxoquinazoline-3-(4 H )-yl) preparation of ethyl acetate
[0046]Add 3.00g (20.53mmol) quinazolin-4-one, 60mL acetone and 3.00g potassium carbonate in sequence to a 100mL single-necked flask, and slowly add 3.85g (22.58mmol) ethyl bromoacetate in aceto...
Embodiment 2
[0056] Example 2: Compound 3-((4-((pyridine-2-methenyl)amino)-5-thione-4,5-dihydro-1 H -1,2,4-triazol-3-yl)methyl)quinazoline-4(3 H )-Synthesis of ketone (Compound No. F2 ):
[0057]
[0058] Steps (1)-(5) are the same as Steps (1)-(5) of Embodiment 1;
[0059] Step (6) was synthesized according to the method and conditions of step (6) in Example 1, except that pyridine-2-carboxaldehyde was used as the raw material and purified after reaction to obtain a dark green solid with a yield of 54.5% m.p.238-240°C. 1 HNMR (DMSO- d 6 ,500MHz) δ :5.47(s,2H),7.57-7.62(m,2H),7.71(d, J =8.00Hz,1H),7.87(t, J =8.00Hz,1H),8.02(t, J =7.60Hz,1H),8.16(t, J =7.60Hz,2H),8.50(s,1H),8.76(d, J =5.00Hz,1H),10.47(s,1H),14.12(s,1H); 13 CNMR (DMSO- d 6 ,125MHz) δ :41.03,121.91,122.12,126.66,127.06,127.86,127.94,135.30,137.90,147.88,148.34,148.52,150.73,151.77,160.51,160.71,162.60.IR(KBr,cm -1 ) v :3443,3031,1696,1617;Anal.CalcdforC 17 h 13 N 7 OS:C,56.19;H,3.61;N,26.98.Found:C,56.58...
Embodiment 3
[0060] Embodiment three: compound 3-((4-((((1 H -indole-3-methenyl)amino)-5-thione-4,5-dihydro-1 H -1,2,4-triazol-3-yl)methyl)quinazoline-4(3 H )-Synthesis of ketone (Compound No. F3 ):
[0061]
[0062] Steps (1)-(5) are the same as Steps (1)-(5) of Embodiment 1;
[0063] Step (6) was synthesized according to the method and conditions of step (6) in Example 1, except that indole-3-carboxaldehyde was used as a raw material, and a yellow solid was obtained after purification after reaction, with a yield of 56.4%. m.p.>250°C. 1 HNMR (DMSO- d 6 ,500MHz) δ :5.42(s,2H),7.17(t, J =7.45Hz,1H),7.28(t, J =7.45Hz,1H),7.53(d, J =8.00Hz,1H),7.58(t, J =8.00Hz,1H),7.70(d, J =8.00Hz,1H),7.87(t, J =7.50Hz,1H),8.11-8.17(m,3H),8.47(s,1H),9.79(s,1H),12.07(s,1H),13.82(s,1H); 13 CNMR (DMSO- d 6 ,125MHz) δ :41.30,110.30,112.90,121.92,122.14,122.64,123.86,124.65,126.65,127.86,127.89,135.25,136.37,137.93,147.25,148.40,1468.47,160.58 -1 ) v :3258,3064,2930,1695,1616;Anal.Calcdfor...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com