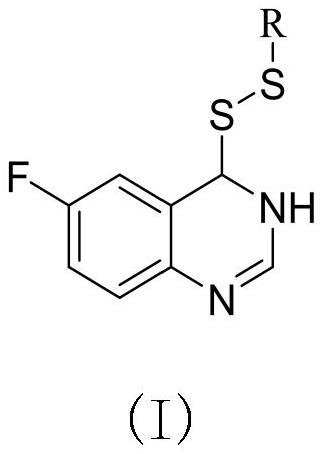
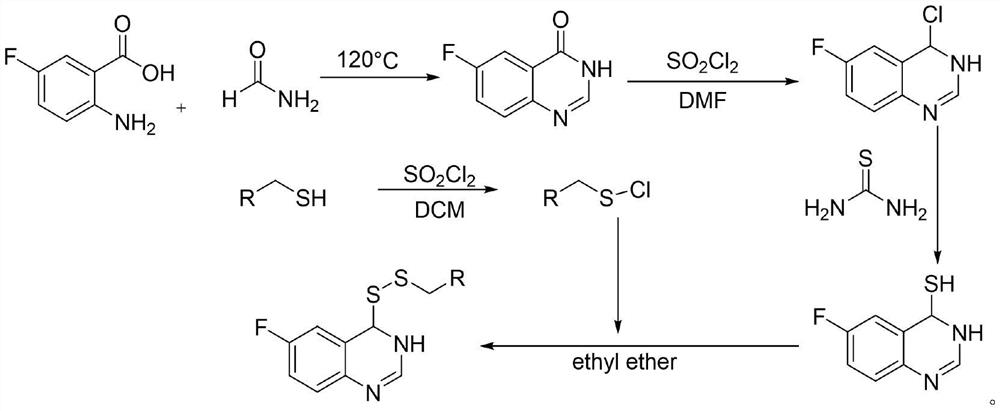
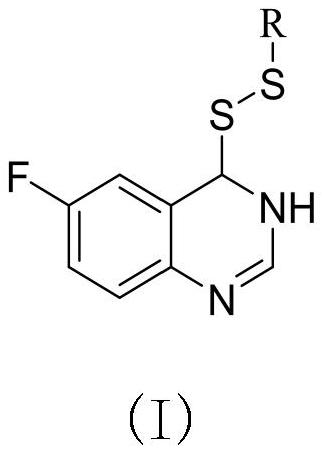
Preparation method and application of 6-fluoroquinazoline derivatives containing disulfide structures
A kind of fluoroquinazoline, disulfide technology, applied in the field of pesticides, can solve the problem of low antibacterial activity and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Example 1: Synthesis of 4- (ethyl di-sulfide) -6-fluorozoline
[0042] (1) Preparation of 6-fluoroquinate-4-sulfhydryl group
[0043] 30 g2-amino 5-fluorobenzoic acid, 60 ml of formamide was added to 150 ml of round bottom flask, and the stirring was raised to 120 ° C, and the reflux reaction was about 5 h, TLC tracking, after the reaction, fell to room temperature, poured into the appropriate amount of ice water The pH was adjusted to a weak alkaline, filtered to give 5-fluofluorozolinone crude. 3.8 g (23.15 mmol) was added to 100 ml round bottom flask, and chlorinated sulfoxide (16.79 mL, 231.51 mmol) was added to stir up to 84 ° C for reflux reaction, and the catalytic amount of DMF was added dropwise during the temperature rise. After 4 hours, the heating was stopped, and most of the solvent was removed, quenched in batches, precipitated the white solid, filtered to give 4-chloro-6-fluoroquinazoline. Take tetrahydrofuran as a solvent, taking 4 g (21.91 mmol) 4-chloro-6-...
Embodiment 2
[0048] Example 2: Synthesis of 4- (propyl di-sulfide ether) -6-fluorozoline
[0049] Preparation of 6-fluoroquinazoline-4-mercapto group Example 1 Example 1; Preparation of propyl apropanine and preparation of 4- (propactiacyl ether) -6-fluoroquanoline Refer to Example 1, of which sulfonyl chloride , The molar ratio of propyl alcohol and 6-fluoroquinazoline-4-mercapto group is 4: 2: 1.
Embodiment 3
[0050] Example 3: Synthesis of 4- (butyl dithiosis ether) -6-fluorozoline
[0051] Preparation of 6-fluoroquinazoline-4-Sulfhydryl group Example 1 Example 1 Example 1 Example 1 Example 1 Preparation of Butyl Sulfonia and Preparation of 4- (Butyl Disborne Eester) -6-Fluoroquanoline Refer to Example 1, of which sulfonyl chloride However, the molar ratio of butylthiol and 6-fluoroquinazoline-4-mercapto group is 4: 2: 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com