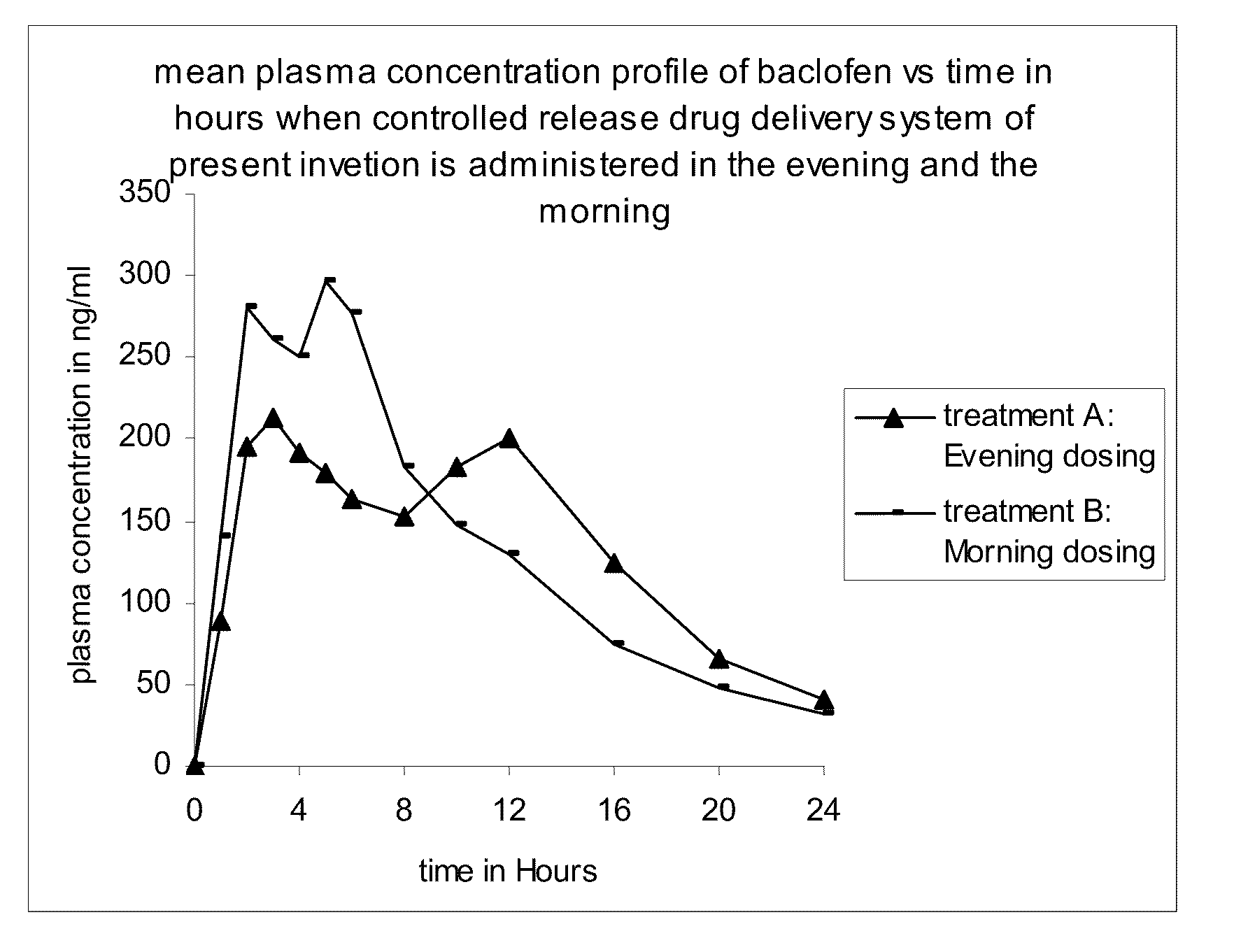
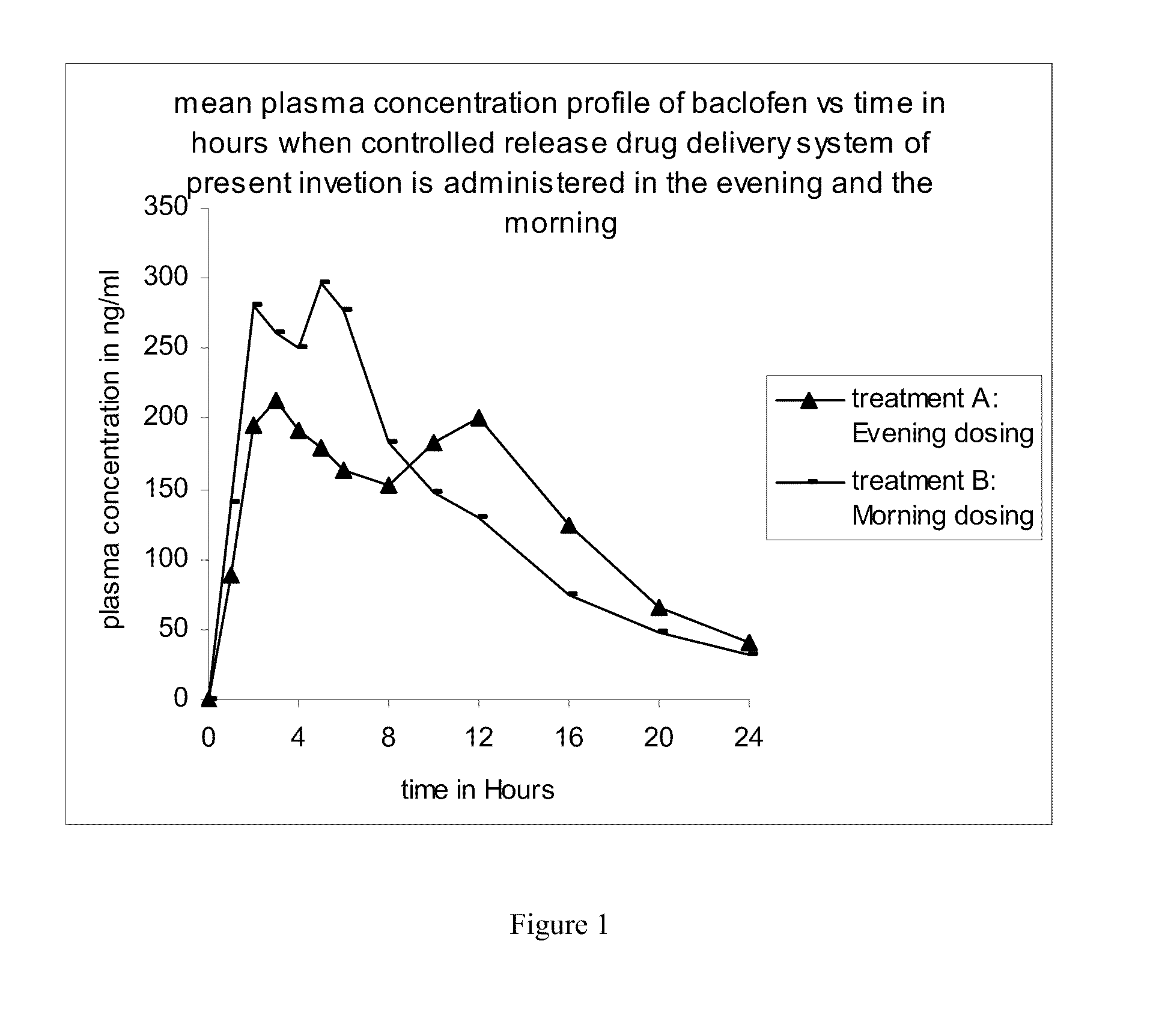
Method of treating a disease condition susceptible to baclofen therapy
a disease condition and effective treatment technology, applied in the field of effective methods of treating a disease condition susceptible to baclofen therapy, can solve the problems of reduced bioavailability of release preparations, increased plasma concentrations, and increased blood plasma concentrations. , to achieve the effect of increasing plasma levels
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0076]This example represents an embodiment of the controlled release drug delivery system which is gastric retention drug delivery system comprising baclofen. It is prepared according to formulae given Table 1 below.
TABLE 1first region of second compositionS.Qty in% by weight of theNo.Ingredientsmgdrug delivery system1Baclofen353.252Mannitol32430.123Hydroxypropyl Cellulose -mw807.431150; apparent viscosity1500-3000 at 1% w / v4Colloidal silicon dioxide50.465Talc12.51.666Magnesium stearate12.51.167Hydrogenated Vegetable oil20.01.868Mannitol46.04.279Water soluble protective20.01.85film coating based on lowviscosity hydroxypropyl-methyl cellulose
[0077]First Region of Second Composition
[0078]Table 1 gives the formula for the preparation of the first region of the second composition. The first region is the formulation that is filled into gelatin capsules. The polymer hydroxypropylmethyl cellulose, low viscosity that does not function as a rate controlling polymer but only serve as a aid ...
example 2
[0088]A gastric retention drug delivery system comprising baclofen was prepared as mentioned in Table 8 below.
TABLE 8Composition detailsmg per weightof drug% by weight of theIngredientsdelivery systemdrug delivery systemFirst region of the second compositionBaclofen22.55.49Fumaric acid10.02.44Mannitol264.564.5Hydroxypropyl cellulose68.016.59Sodium bicarbonate30.07.32Colloidal silicon dioxide5.01.22Talc5.01.22Magnesium stearate5.01.22Second region of the second compositionAlginic acid54.35Coated to aSodium bicarbonate10.87weight gain ofSodium starch glycolate27.17about 25% byMannitol13.59weight of the corePolyvinylpyrrolidone16.30Talc3.26Polysorbate1.09Third compositionPolycarbophil4.41Coated to aSodium bicarbonate8.82weight gain ofMethacrylic acid copolymer35.29about 18% byEudragit S-1008.82weightMannitol35.29Sodium starch glycolate10.29Polysorbate0.59Polyethylene glycol1.47Talc2.35Diethyl phthalate6.62First compositionBaclofen7.5Coated to aPolyvinylpyrrolidone1.50weight gain ofTalc...
example 3
[0090]A gastric retention drug delivery system comprising baclofen was prepared as mentioned in Table 9 below.
TABLE 9Quantity(% w / w of the drugIngredients(mg / capsule)delivery systemFirst region of the second compositionBaclofen22.55.49Fumaric acid10.02.44Mannitol200.548.90Polycarbophil108.026.34Sodium bicarbonate54.013.17Colloidal silicon dioxide5.01.22Talc5.01.22Magnesium stearate5.01.22
[0091]The first region of the second composition was obtained by blending the excipients listed in Table 10 with baclofen and filling it in a hard gelatin capsule. The capsule was then coated with a second region of the second composition and third composition similar to example 2 described above.
PUM
| Property | Measurement | Unit |
|---|---|---|
| time | aaaaa | aaaaa |
| time | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com