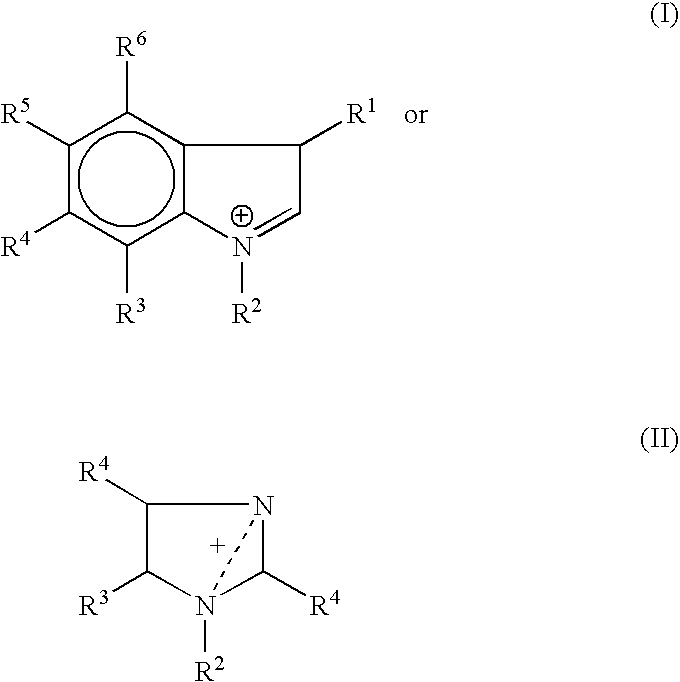
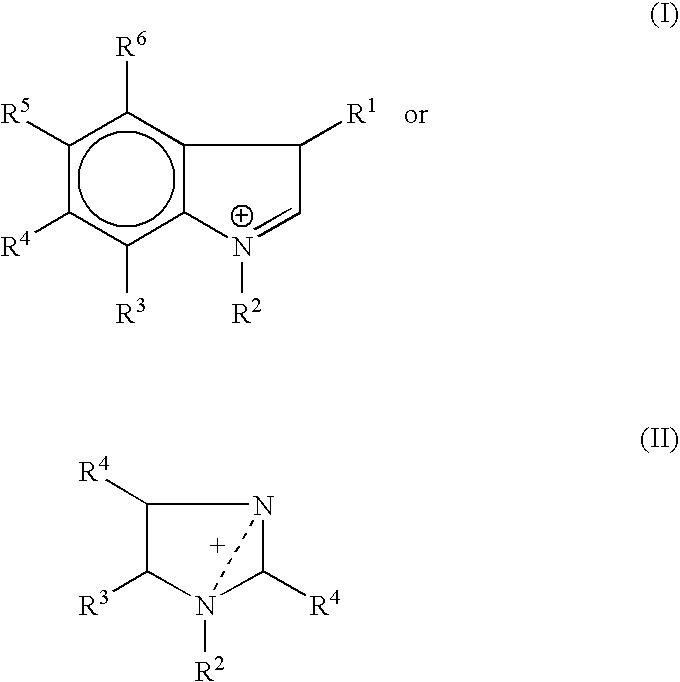
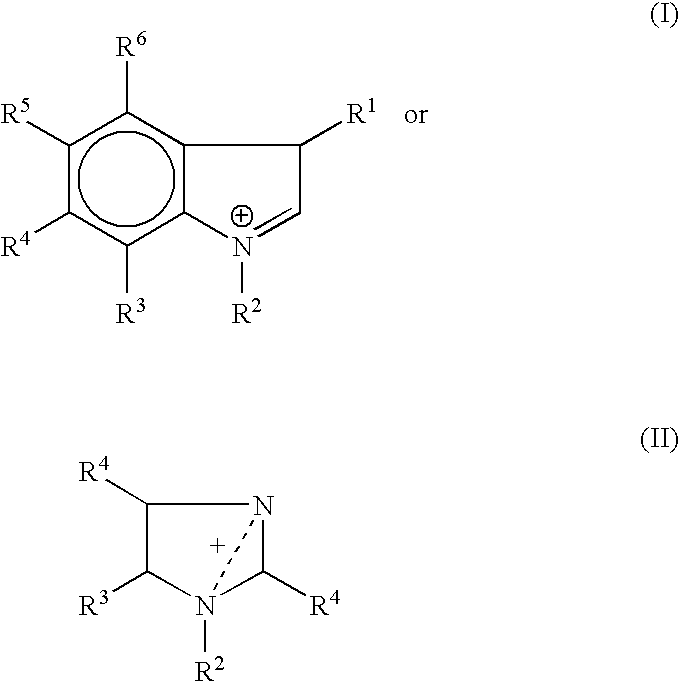
Zwitterion solution for low-volume therapeutic delivery
a zwitterion solution and low-volume technology, applied in the field of high-concentration therapeutic zwitterion solutions, can solve the problems of poor solubility of zwitterion therapeutic agents in saline solution, limited pharmokinetics of agents, low dose, etc., and achieve the effect of increasing concentration or ease of solution and/or storag
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0035]Multivalent physiologic ion solution formation. A solution (A) is produced with 8.66 g NaCl, 0.224 g KCl, 0.206 g CaCl2.2H20 and 0.163 g MgCl2.6H20 dissolving in 500 mL of deionized water. A solution (B) is produced with 0.214 g Na2HPO4.7H20 and 0.027 g NaH2PO4.H20 dissolving in 450 mL of water. The pH is adjusted to 6.0, 6.5, 7.0, 7.3, 7.6 and 8.0 as necessary with either NaOH or H3PO4 and dilution to final volume of 500 mL with deionized water. A final multivalent physiologic ion solution is obtained by mixing equal parts of Solution A and B. pH is tested and adjusted to the desired final pH if necessary. All reagents are available from sources known in the art. Illustratively, reagents are available from Sigma-Aldrich Corp., St. Louis, Mo.
example 2
[0036]Ofloxacin solubilization in multivalent physiologic ion solution. Ofloxacin powder, or ground tablets, are weighed and added to accurately measured volumes of MPI solution composed of 20 mM sodium carbonate, 8 mM MgCl2.6H2O, 5 mM CaCl2, 150 mM NaCl titrated to pH 7.0. Solutions are warmed to 37° C. in a water bath. Each solution is manually mixed every minute during the first five minutes and at five minute intervals thereafter for an entire 30 minute aliquot pull period. Visually clear solutions are immediately obtained, but gentle stirring or vortexing is applied to the samples for several seconds to ensure complete solubilization of the ofloxacin. Clear solutions are achieved with no visual particulate matter remaining. Approximate 5 mL aliquots are pulled at 2, 5 and 30 minutes of incubation using a syringe equipped with a 10 micron filter tip. The aliquots are analyzed by high performance liquid chromatography (HPLC). For analyses, 2.0 mL of each aliquot is transferred to...
example 3
[0037]Ofloxacin solubilization in artificial cerebrospinal fluid. Ofloxacin powder, or ground tablets, are weighed and added to accurately measured volumes of aCSF. Visually clear solutions are immediately obtained, but gentle stirring or vortexing is applied to the samples for several seconds to ensure complete solubilization of the ofloxacin. Ofloxacin concentrations are achieved in aCSF at 10 mg / ml. Solutions with greater than 7 mg / ml final concentrations required gentle agitation for 2 minutes or less. Clear solutions are achieved with no visual particulate matter remaining.
PUM
| Property | Measurement | Unit |
|---|---|---|
| solubility | aaaaa | aaaaa |
| solubility | aaaaa | aaaaa |
| concentrations | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com