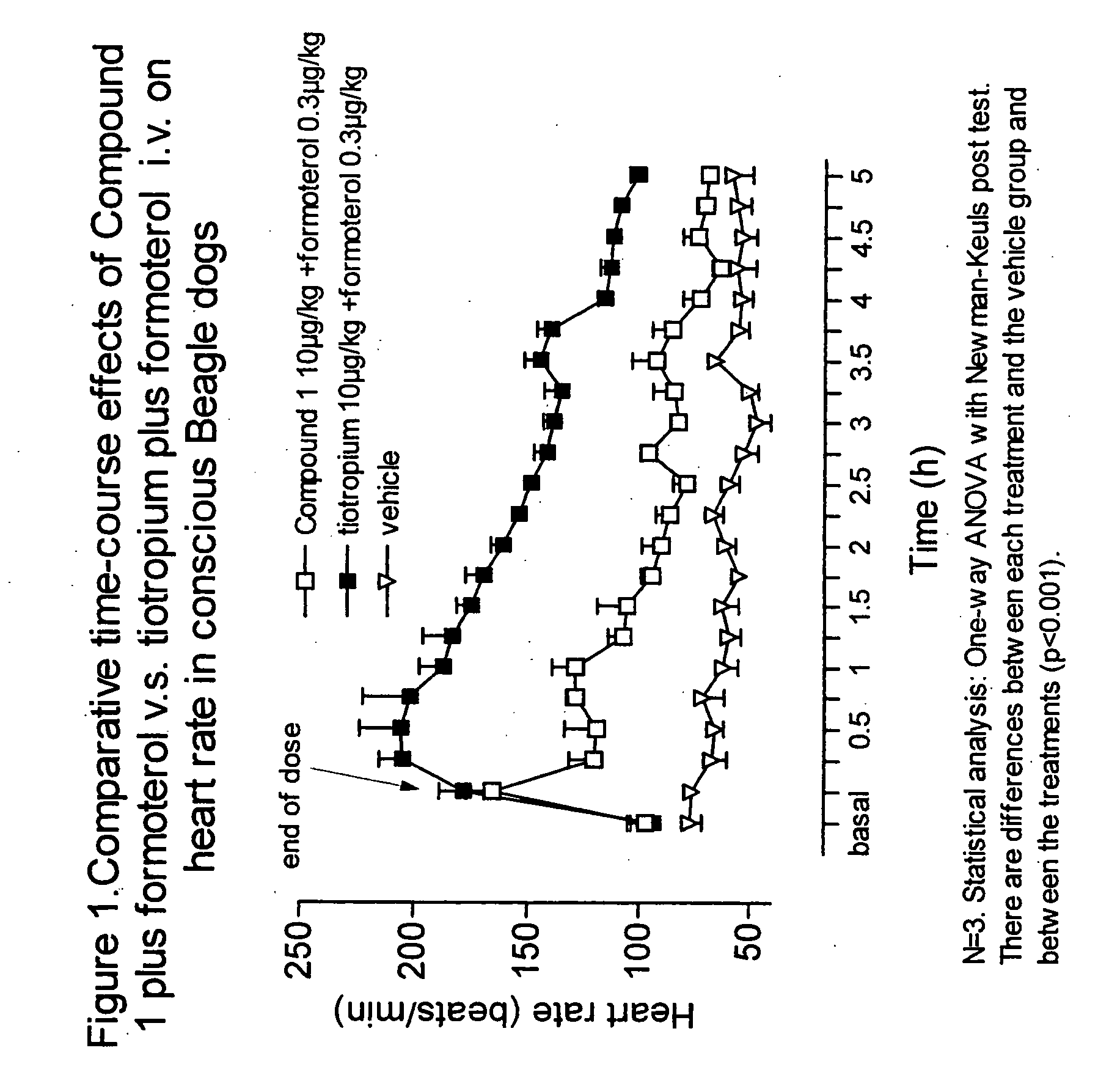
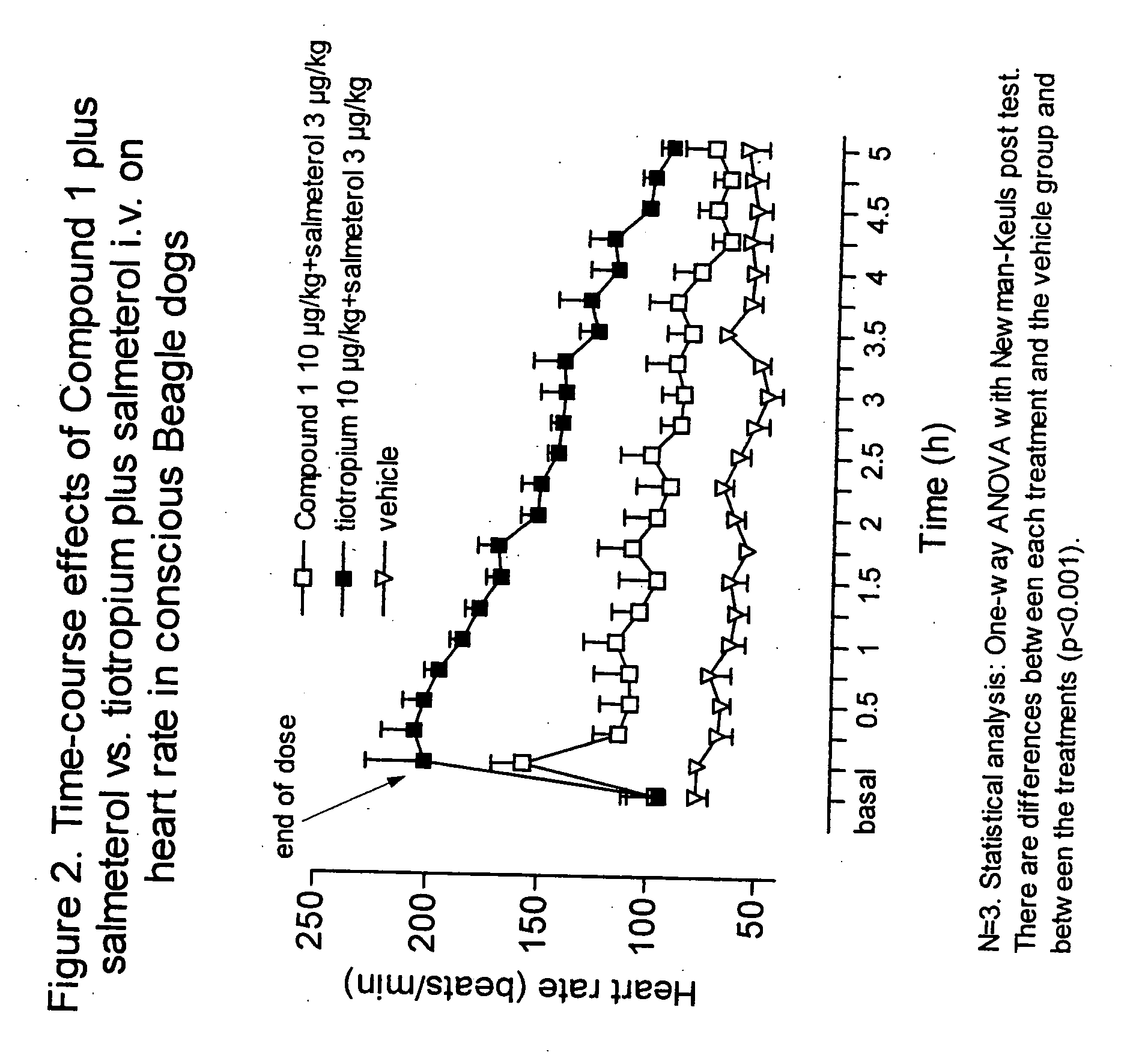
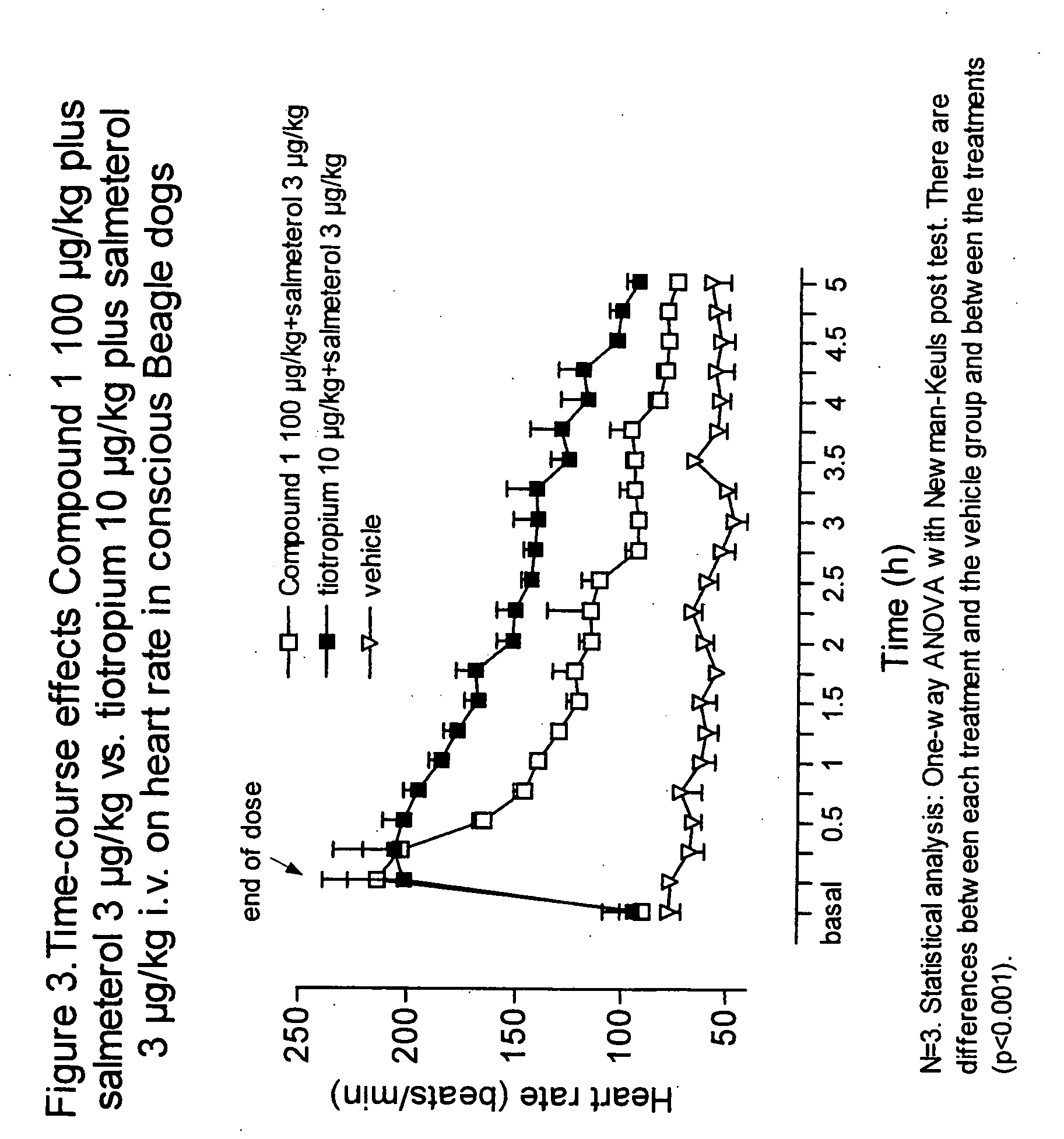
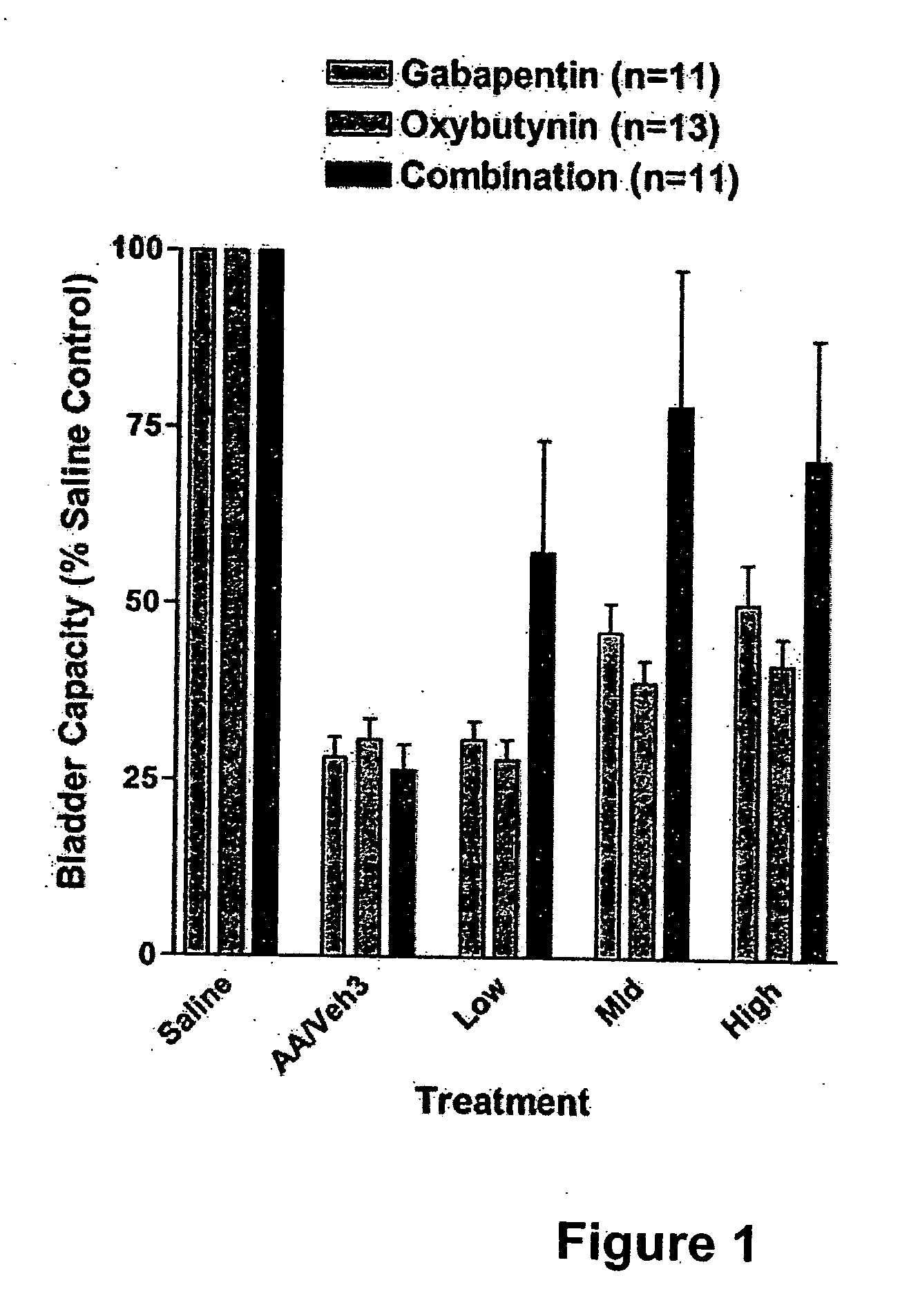
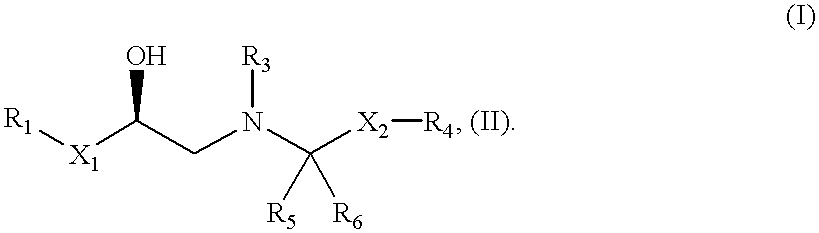Patents
Literature
111 results about "Adrenergic agonist" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
An adrenergic agonist is a drug that stimulates a response from the adrenergic receptors. The five main categories of adrenergic receptors are: α₁, α₂, β₁, β₂, and β₃, although there are more subtypes, and agonists vary in specificity between these receptors, and may be classified respectively. However, there are also other mechanisms of adrenergic agonism. Epinephrine and norepinephrine are endogenous and broad-spectrum. More selective agonists are more useful in pharmacology.
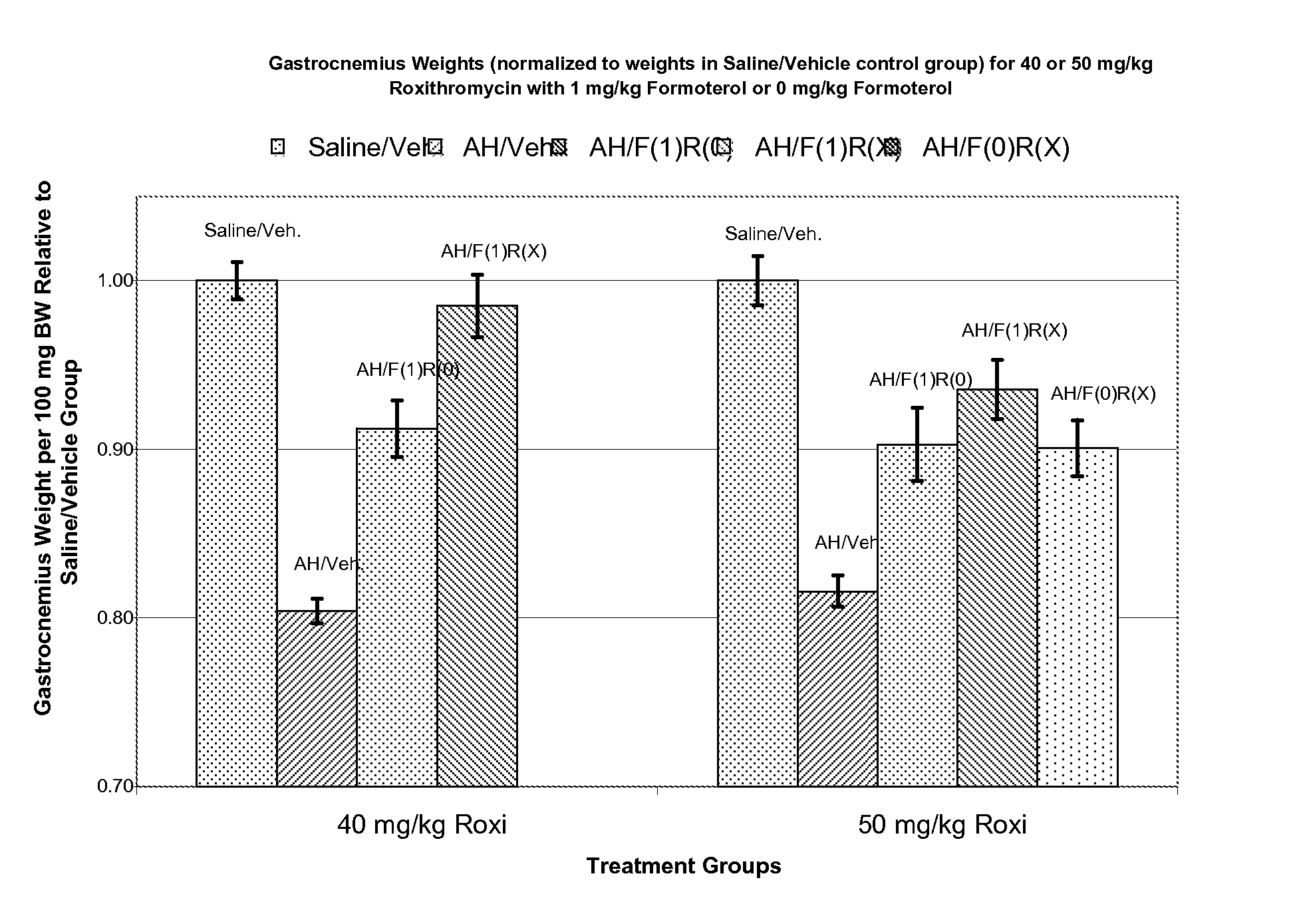
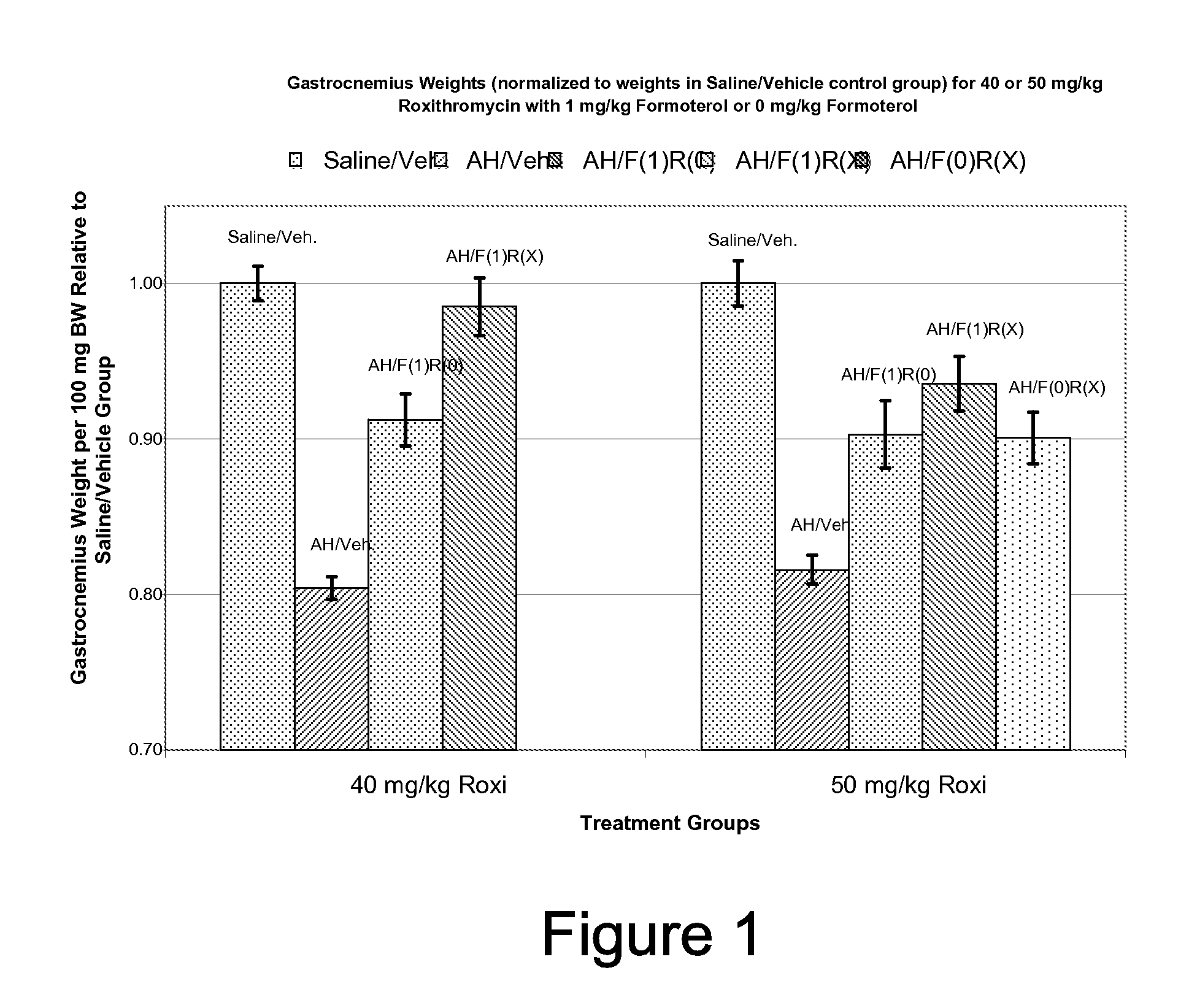
Methods and compositions for the prevention of tolerance to medications
InactiveUS6235725B1Avoid toleranceEasy to managePowder deliveryBiocideTolerabilityAdrenergic receptor agonists
The present invention pertains to the identification of moieties and methods of using the same for preventing tolerance to bronchodilators. More specifically, the present invention pertains to the identification of compositions and methods which are capable of preventing tolerance to beta2-adrenergic agonists. The methods and compositions according to the invention are also useful as analytical tools for functional studies and as combination therapeutic tools.
Owner:TEVA WOMENS HEALTH RES INC
Delivery of a sympatholytic cardiovascular agent to the central nervous system to counter heart failure and pathologies associated with heart failure
A sympatholytic cardiovascular agent delivered by a drug delivery pump to a central nervous system site to alleviate symptoms and otherwise treat heart failure (HF) and pathologies associated with HF. The drug delivery pump can be external or implantable infusion pump (IIP) coupled with a drug infusion catheter extending to the site. A patient activator can command delivery of a dosage and / or an implantable heart monitor (IHM) coupled with a sensor can detect physiologic parameters associated with HF (or pathologies associated with HF) and trigger dosage delivery. The IIP and IHM can be combined into a single implantable medical device (IMD) or can constitute separate IMDs that communicate by any of known communication mechanisms. The sympatholytic cardiovascular agent is one of the group consisting of an alpha-adrenergic agonist and an alpha2-adrenergic agonist, e.g., clonidine, p-aminoclonidine, guanabenz, lidamidine, tizanidine, moxonidine, methyldopa, xylazine, guanfacine, detomidine, medetomidine, and dexmedetomidine.
Owner:MEDTRONIC INC
Drug delivery to the anterior and posterior segments of the eye using eye drops
InactiveUS20110104155A1Detection is simple and fastBiocideSenses disorderAdrenergic DrugsAdrenergic Agent
A method and means for delivery of drugs to the chorio-retina and the optic nerve head which comprises contacting the surface of the eye with an effective amount of drug for treatment of chorio-retina and optic nerve head and a physiologically acceptable adrenergic agent for enhancing delivery of the drug to these tissues in an ophtalmologically acceptable carrier, said adrenergic agent being selected from the group consisting of alpha adrenergic agonist agents, derivatives of the alpha adrenergic agonist agents, beta-blocking agents, derivatives of the beta-blocking agents and mixtures thereof.
Owner:RAOUF REKIK
Pharmaceutical Compositions and Related Methods of Treatment
InactiveUS20080021074A1Decrease and prevent sleepinessDecrease and prevent and lethargyBiocideNervous disorderAdrenergic receptor agonistsClumsiness
Pharmaceutical compositions comprising at least one alpha2-adrenergic agonist or baclofen and at least one alpha1-adrenergic agonist are disclosed. Pharmaceutical compositions comprising tizanidine and modafinil are disclosed. Methods for reducing somnolence, sleepiness, lethargy, dizziness, drowsiness, somnolence, tiredness, lightheadedness, increased weakness, confusion, unsteadiness, clumsiness, or a combination of the symptoms thereof in a human patient; treating pain; and attenuating muscle spasticity, using pharmaceutical compositions comprising at least one alpha2-adrenergic agonist or baclofen and at least one alpha1-adrenergic agonist are disclosed.
Owner:QUESTCOR PHARMA
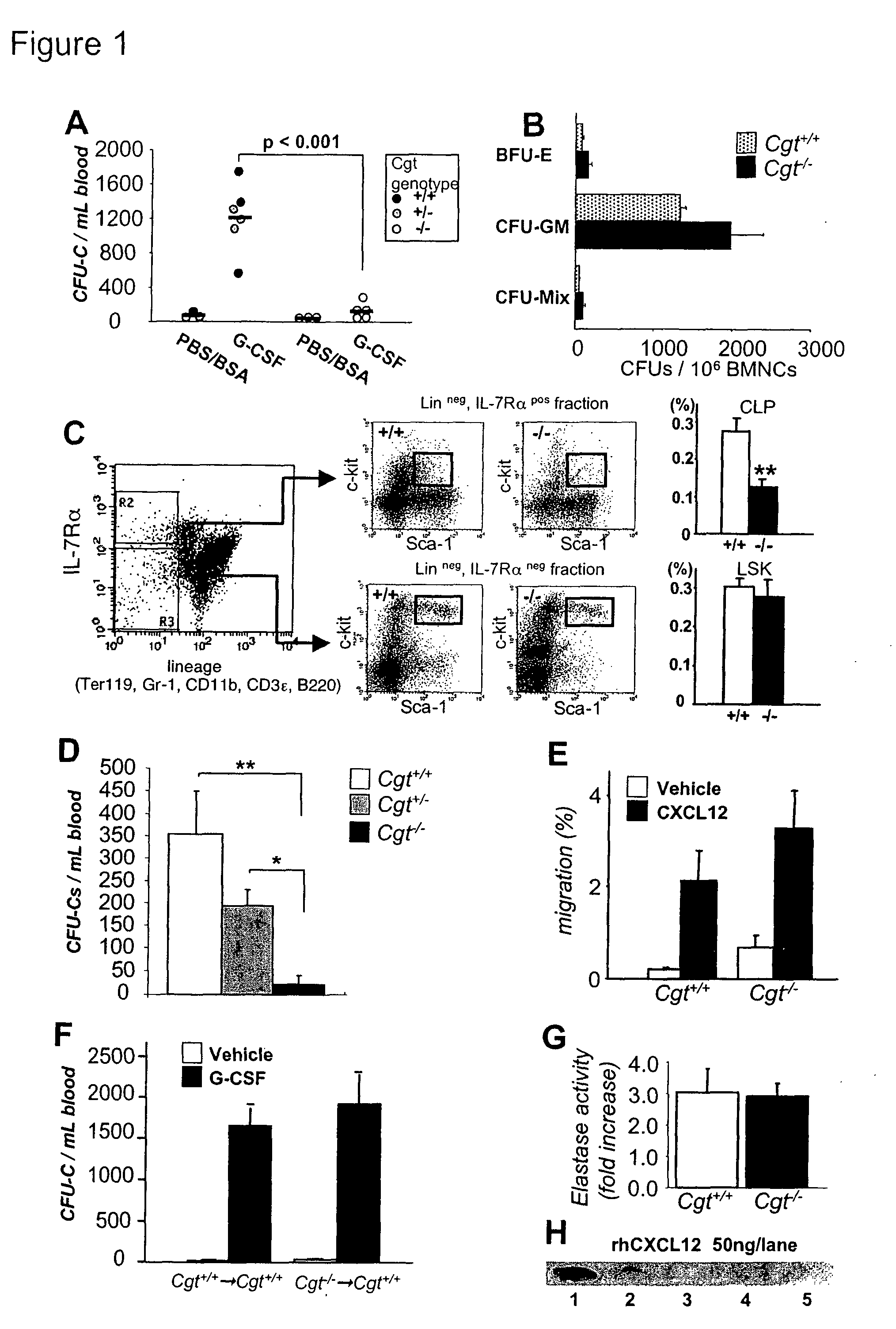
Methods and compositions for modulating the mobilization of stem cells
InactiveUS20070190023A1Enhance mobilizationPrevent egressBiocidePeptide/protein ingredientsDiseaseCXCR4
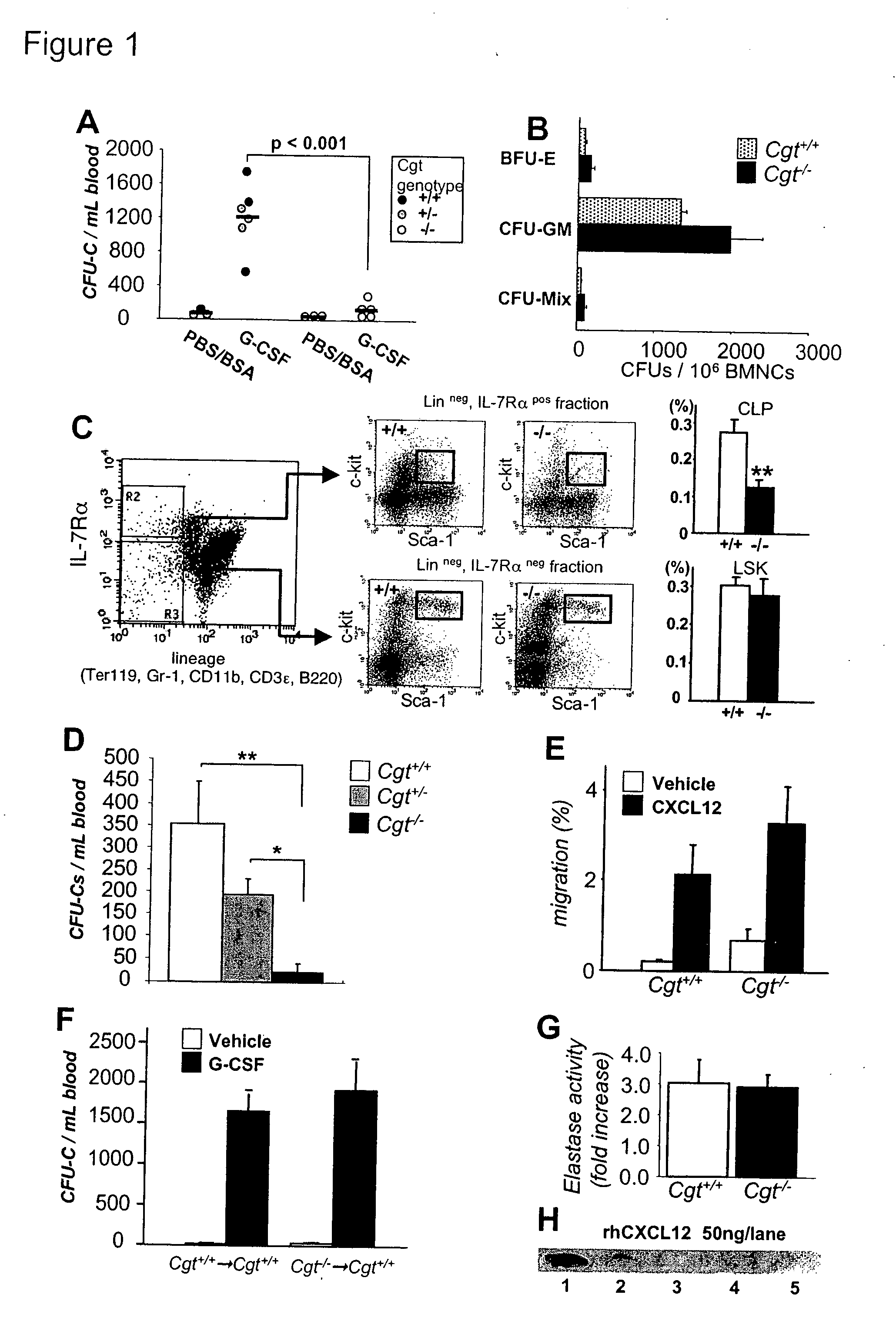
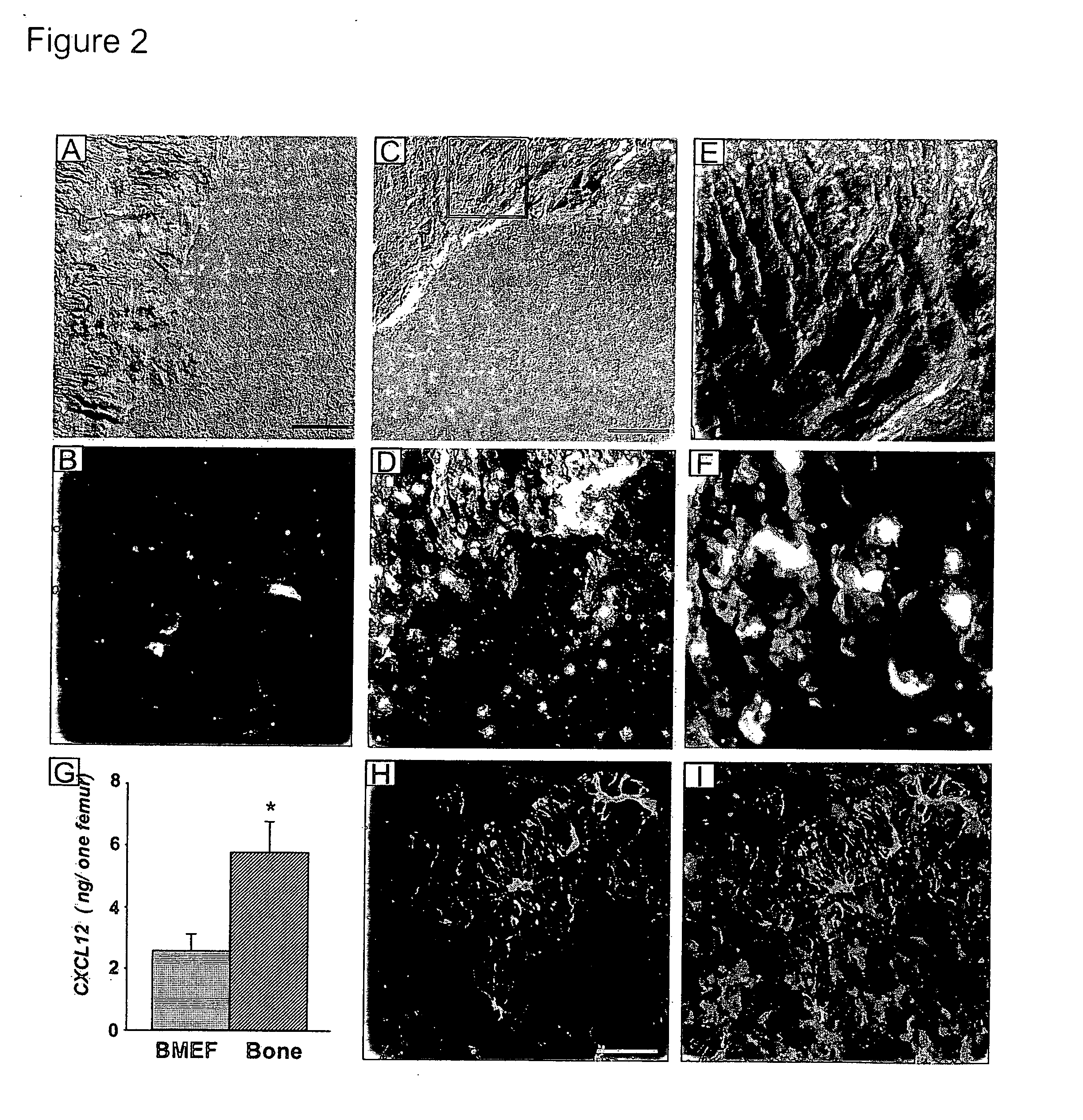
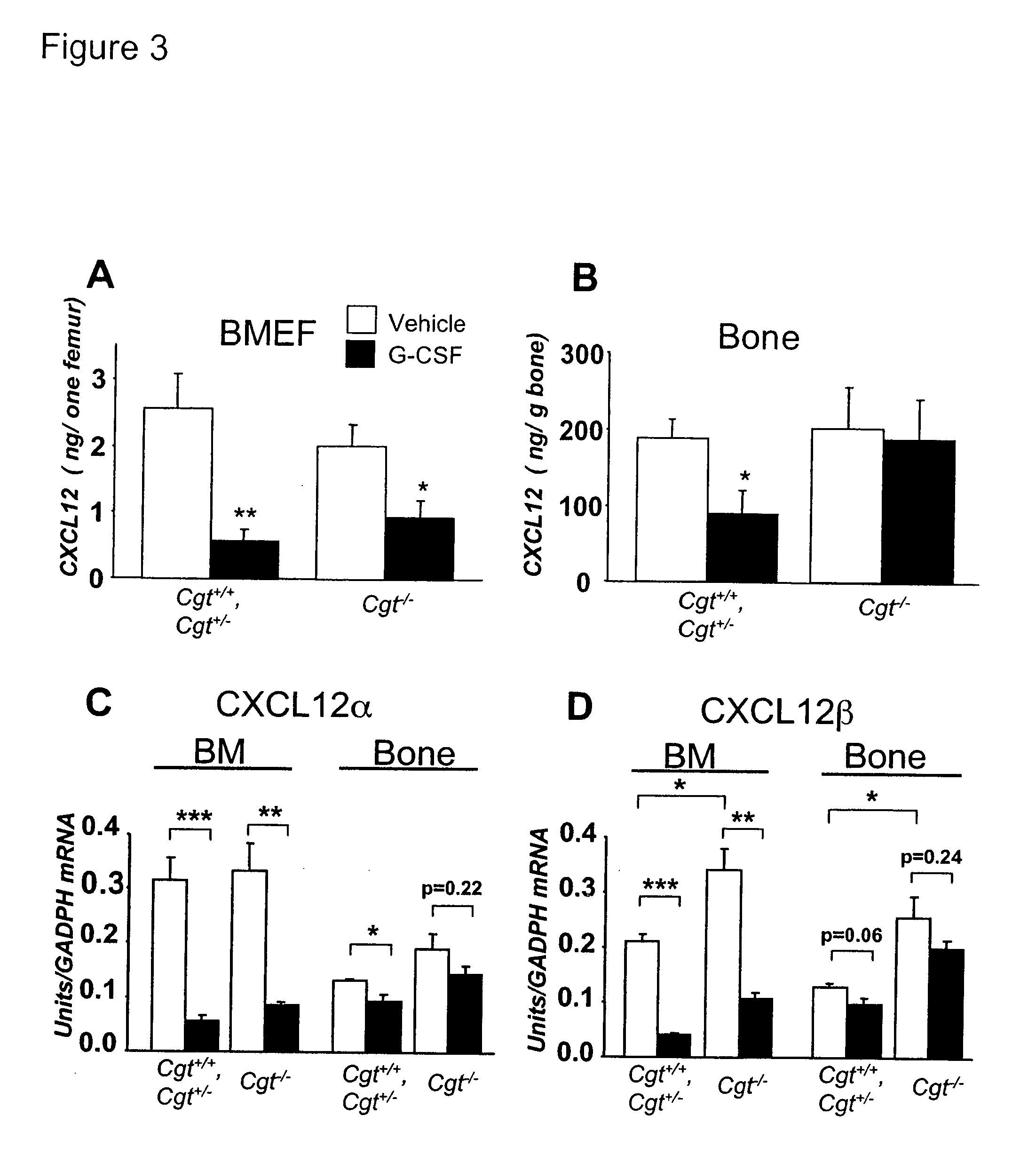
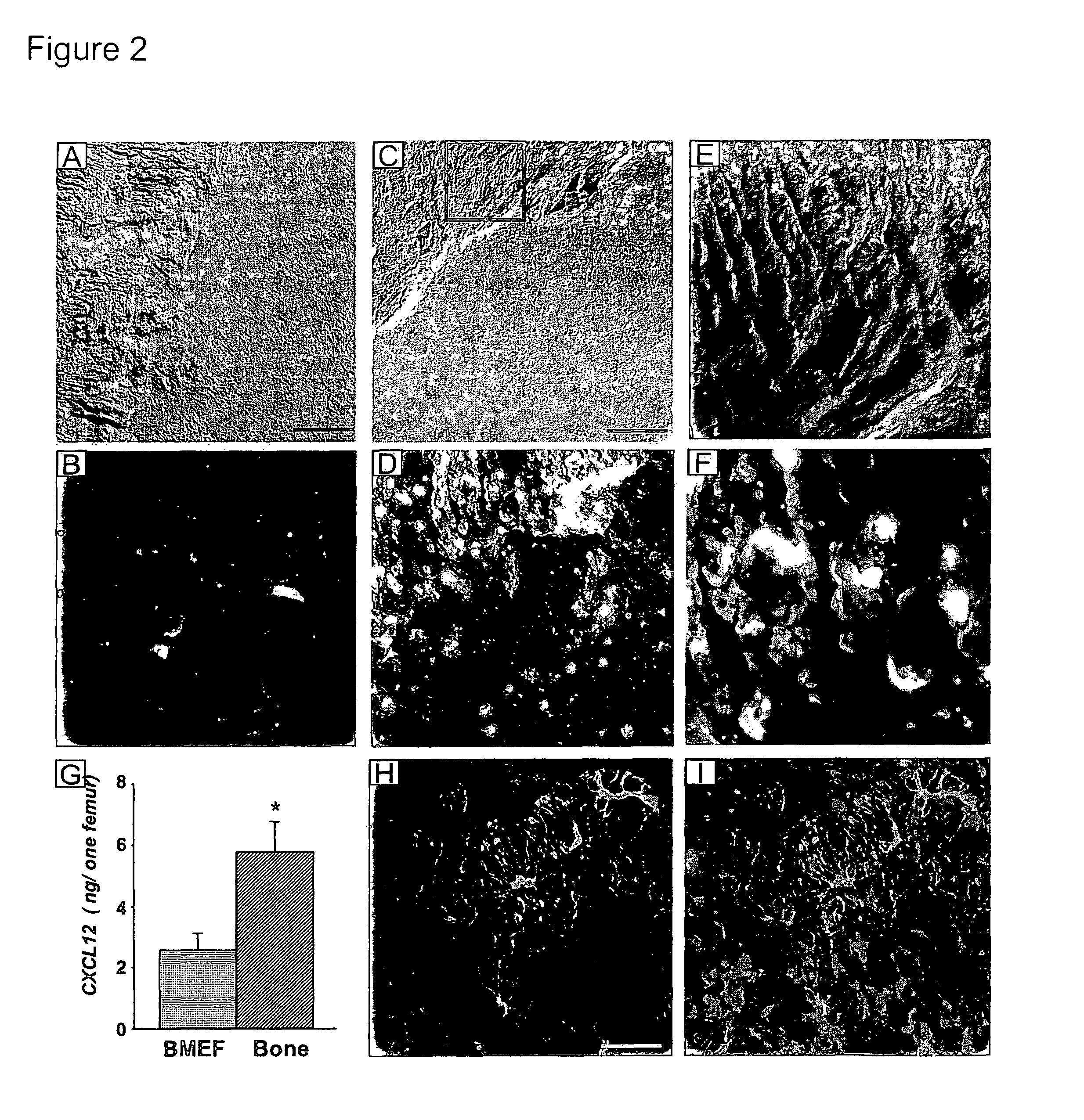
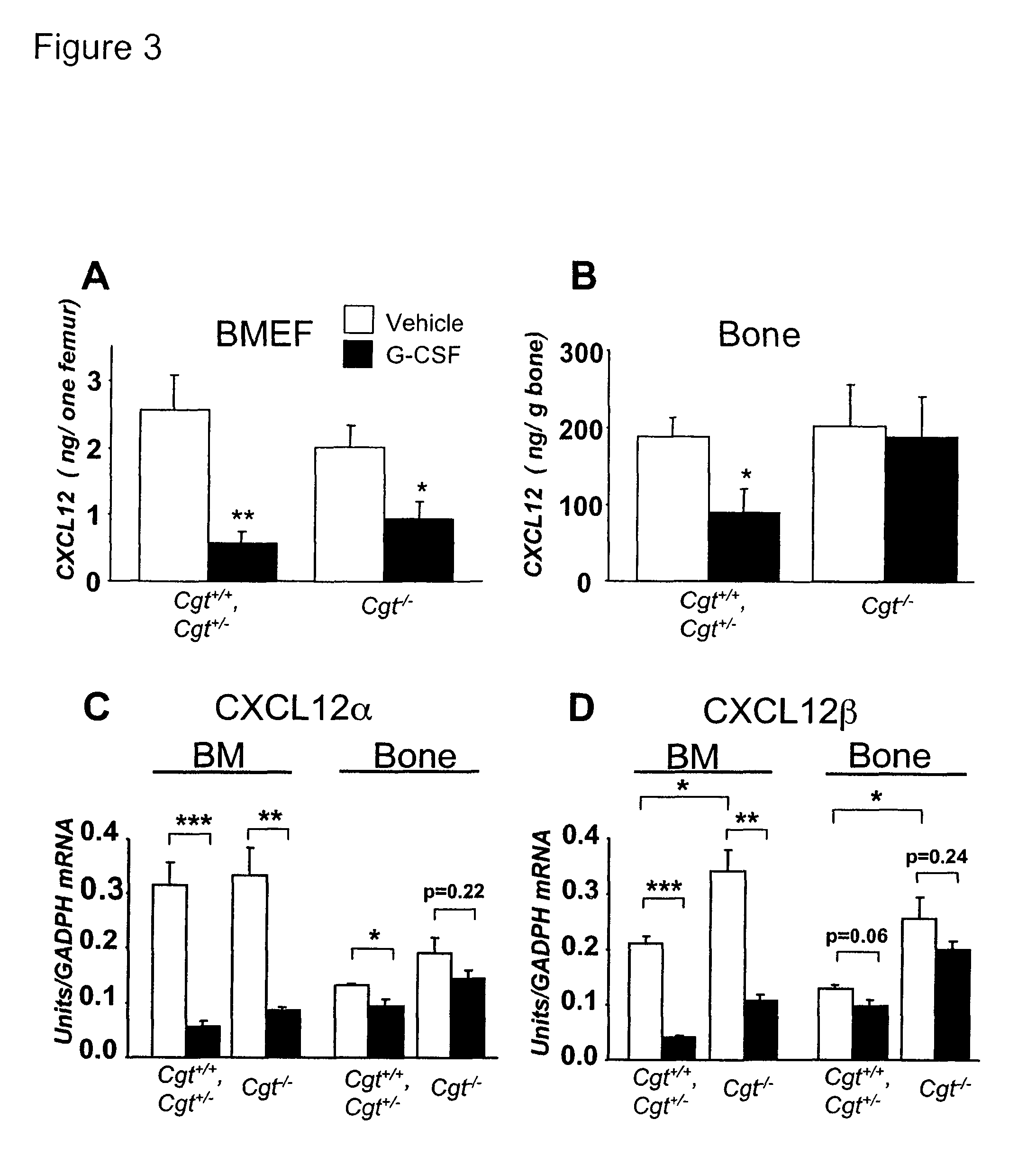
Methods and compositions for modulating the mobilization of stem cells, particularly for promoting or increasing the mobilization of hematopoietic stem cells from the bone marrow to the peripheral blood are disclosed. In particular, the invention relates to the use of adrenergic agonists that act in concert with a mobilization compound or agent. The mobilization agent(s) may act to decrease the expression or function of the chemokine, CXCL12, or may act to block or antagonize CXCR4. The invention also relates to methods of using these compounds or agents for enhancing the mobilization of hematopoietic stem cells when harvesting of the stem cells is necessary for the treatment of diseases, disabilities or conditions whereby transplantation of such cells would be beneficial in ameliorating the symptoms associated with such diseases, disabilities or conditions. Methods of screening for novel agents and pharmaceutical compositions comprising these agents are also disclosed.
Owner:MT SINAI SCHOOL OF MEDICINE
Combinations comprising antimuscarinic agents and beta-adrenergic agonists
Combinations comprising (a) a β2 agonist and (b) an antagonist of M3 muscarinic receptors which is 3(R)-(2-hydroxy-2,2-dithien-2-ylacetoxy)-1-(3-phenoxypropyl)-1-azoniabicyclo[2.2.2]octane, in the form of a salt having an anion X, which is a pharmaceutically acceptable anion of a mono or polyvalent acid are useful, e.g., for the treatment of respiratory disease, e.g., asthma or chronic obstructive pulmonary disease.
Owner:GRAS ESCARDO JORDI +3
Methods for treating pain using smooth muscle modulators and a2 subunit calcium channel modulators
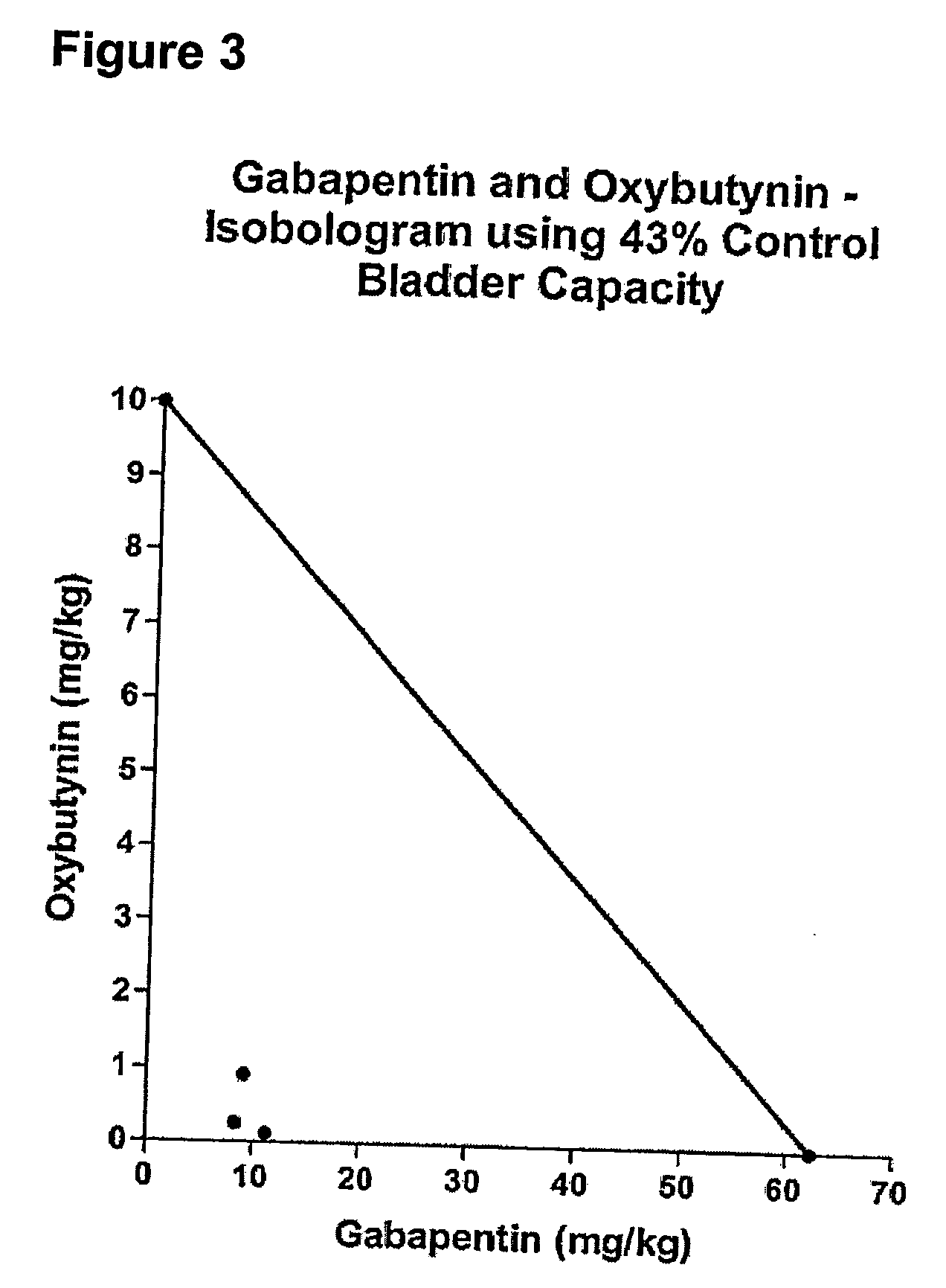
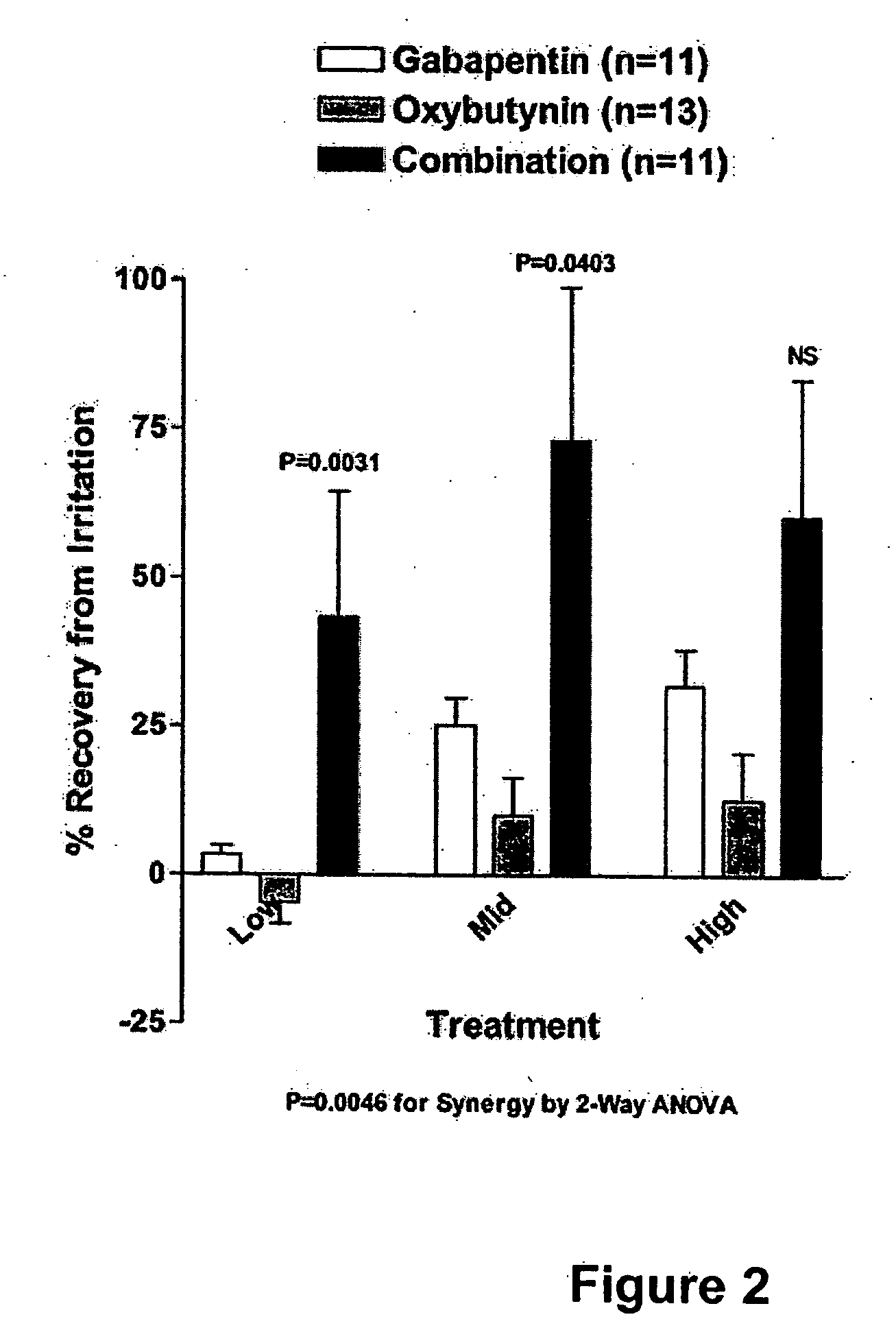
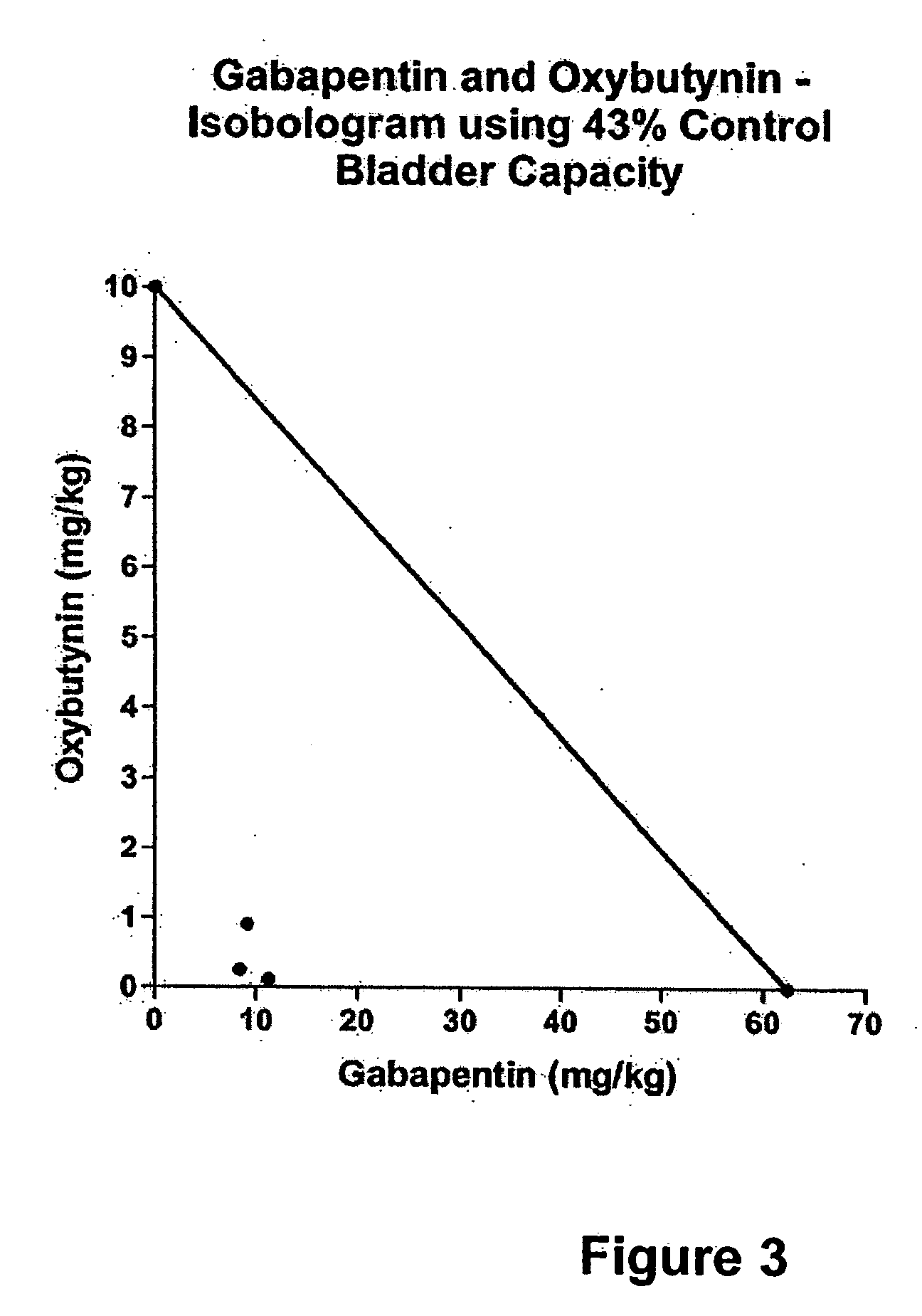
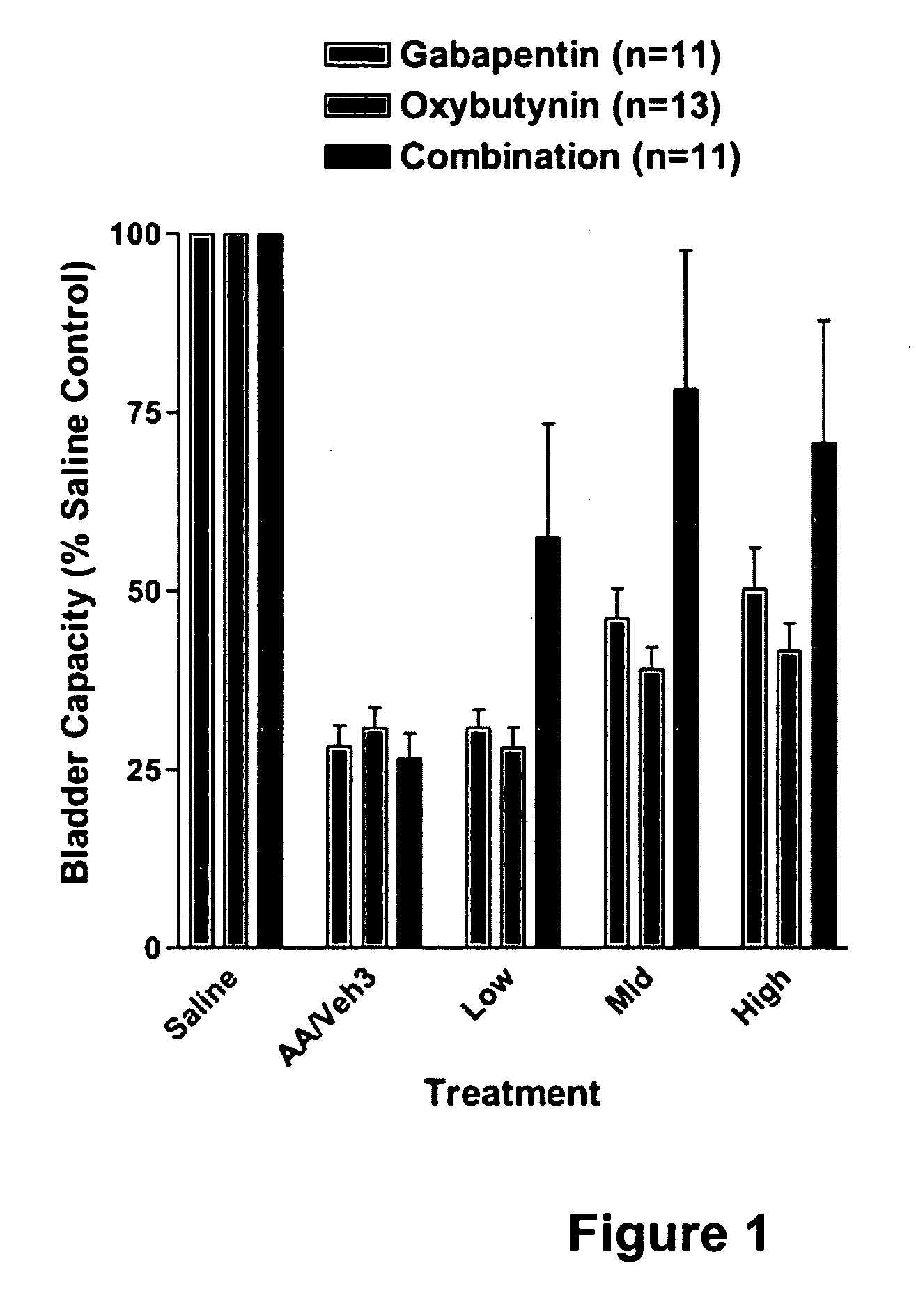
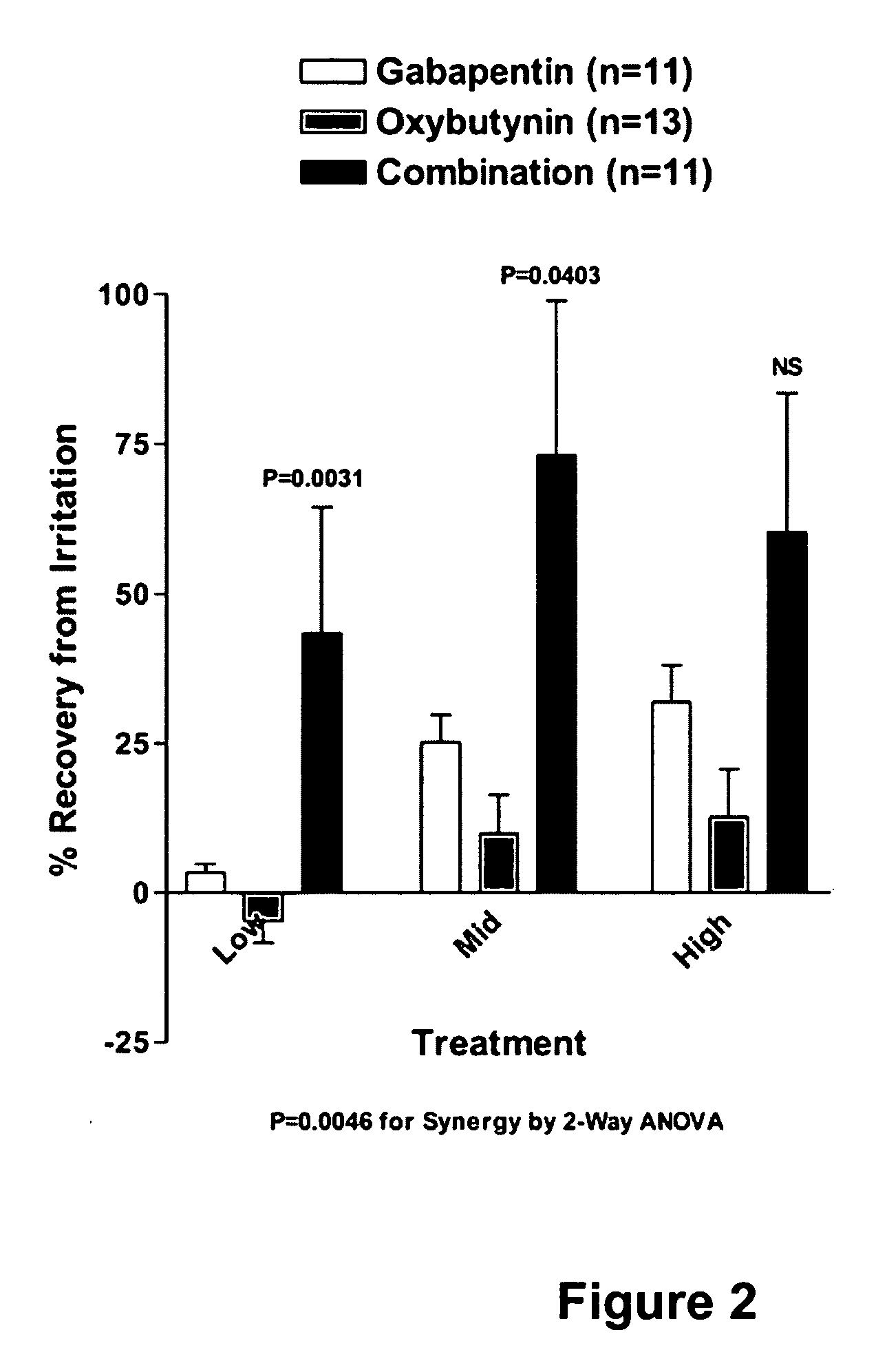
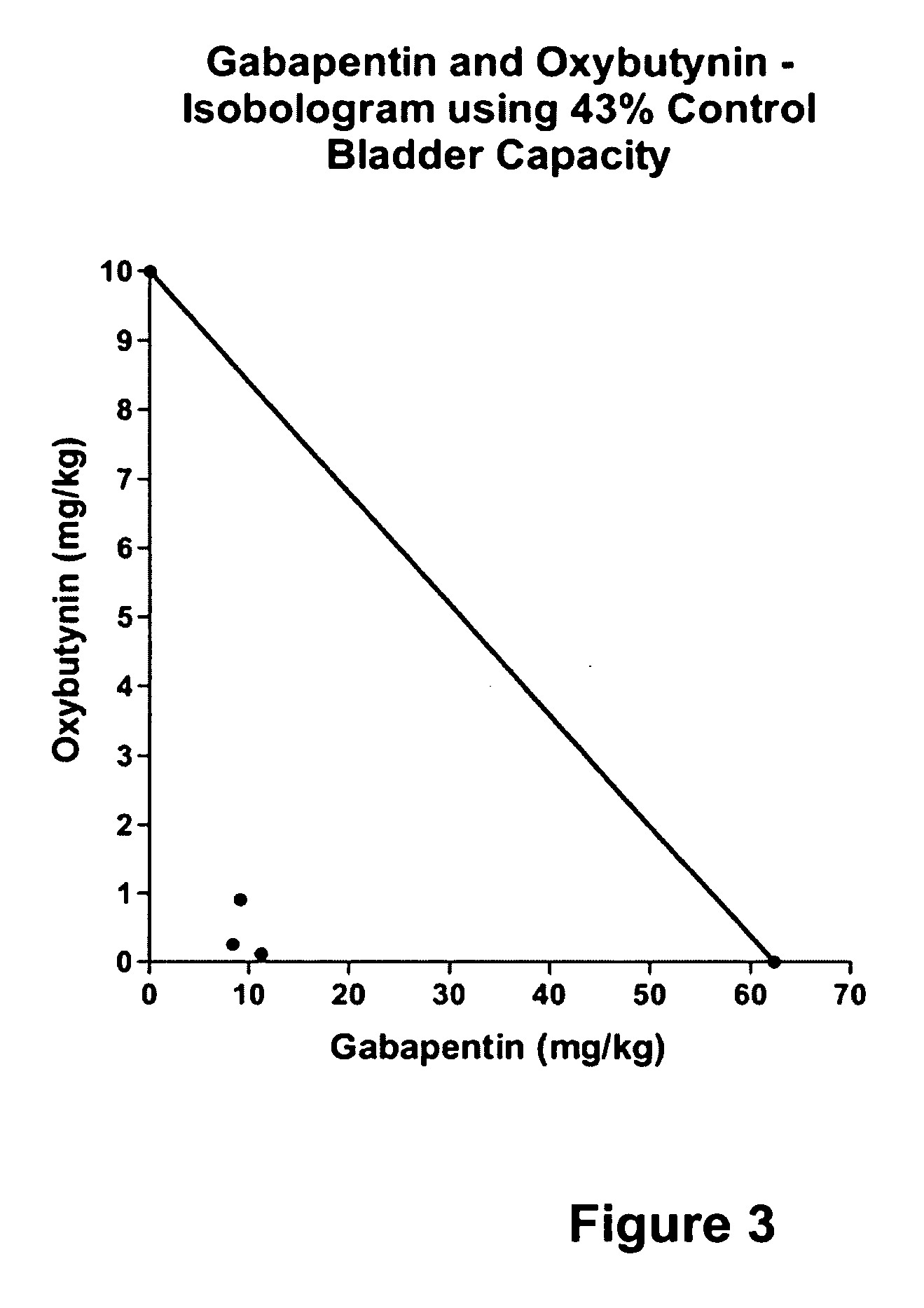
InactiveUS20060264509A1Limited efficacyReduce patient complianceBiocideOrganic active ingredientsGabapentinAdrenergic receptor agonists
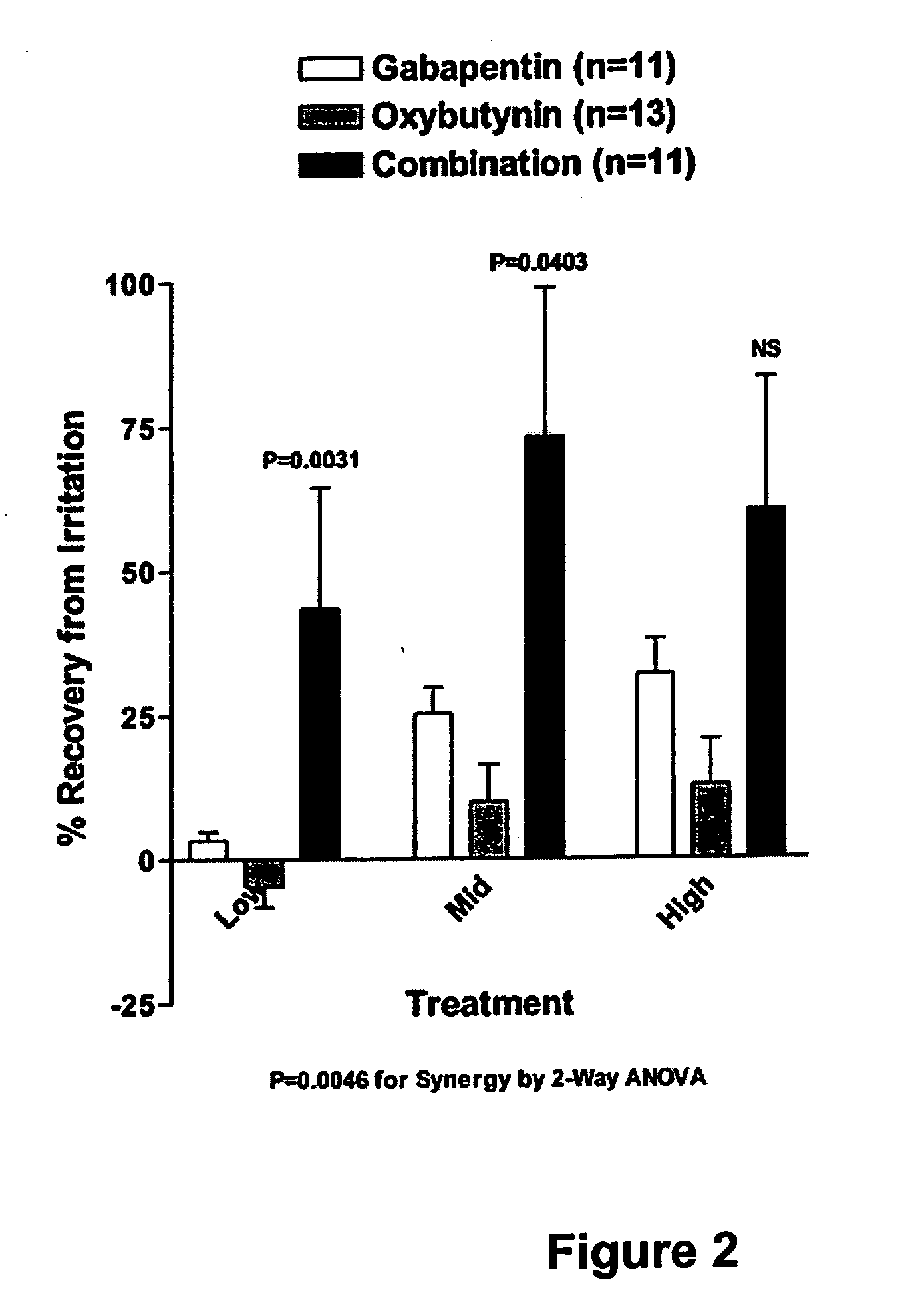
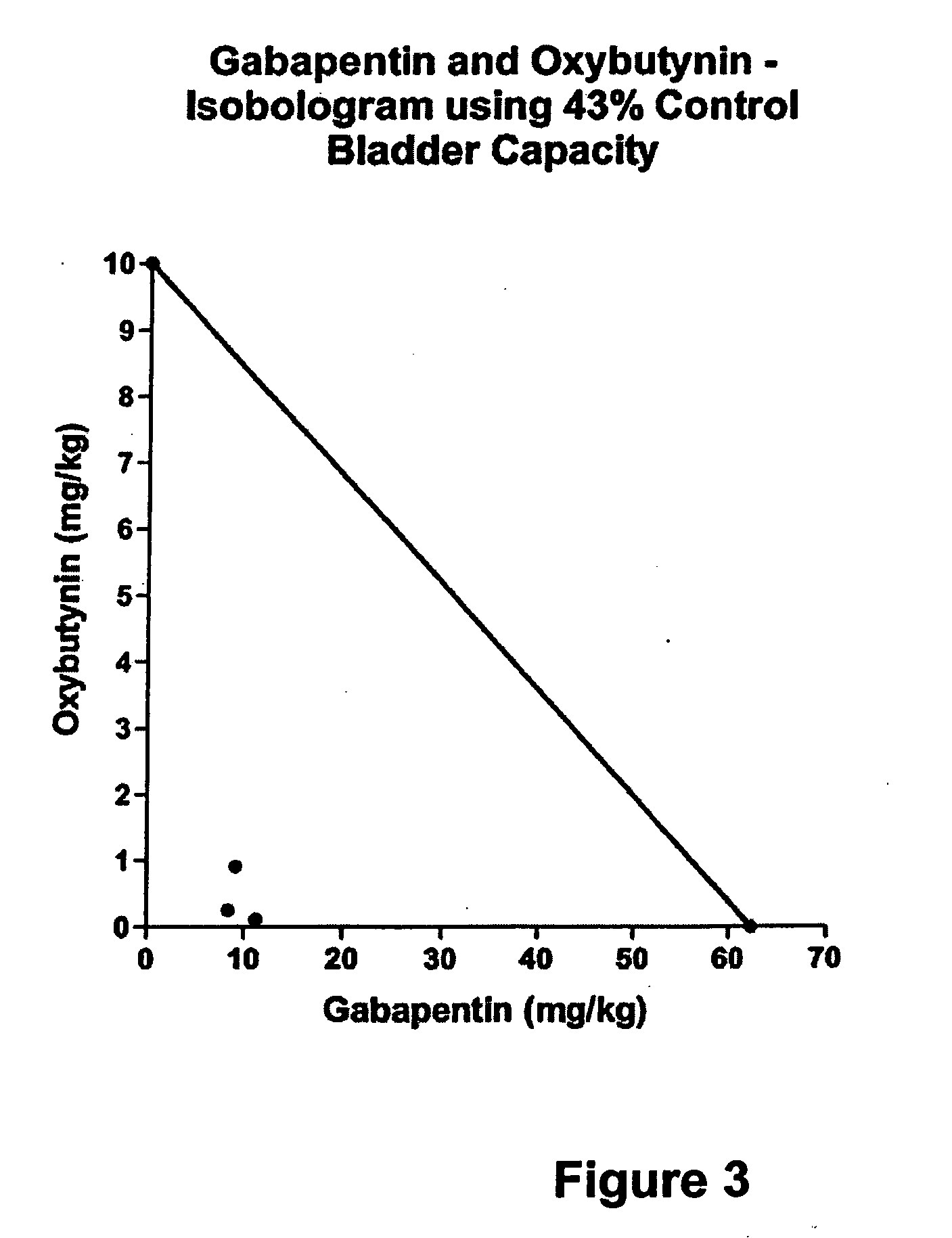
A method is provided for using α2δ subunit calcium channel modulators or other compounds that interact with the α2δ calcium channel subunit in combination with one or more compounds with smooth muscle modulatory effects to treat pain. According to the present invention, α2δ subunit calcium channel modulators include GABA analogs (e.g., gabapentin and pregabalin), fused bicyclic or tricyclic amino acid analogs of gabapentin, and amino acid compounds. Compounds with smooth muscle modulatory effects include antimuscarinics, β3 adrenergic agonists, spasmolytics, neurokinin receptor antagonists, bradykinin receptor antagonists, and nitric oxide donors.
Owner:DYNOGEN PHARM INC
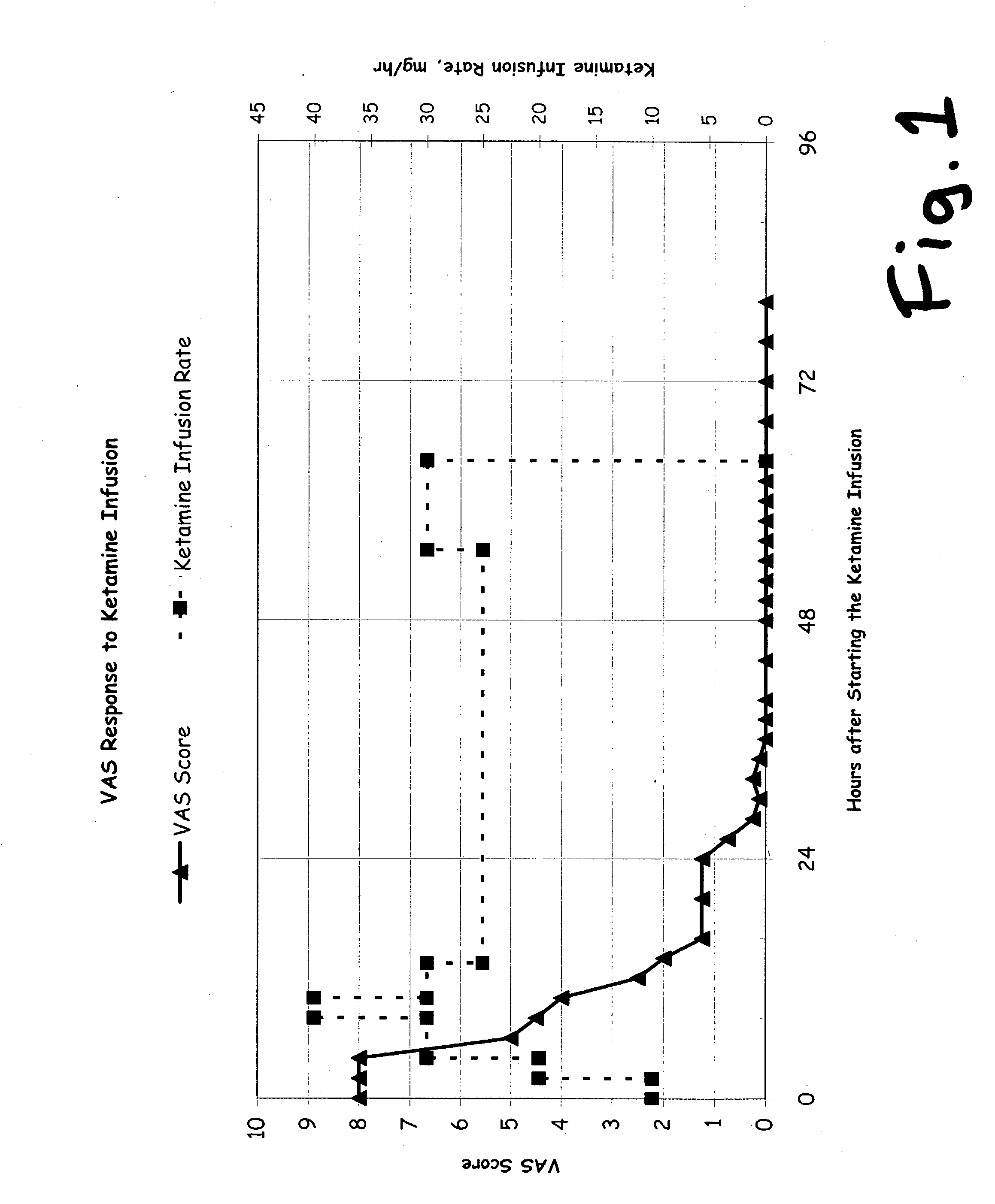
Prolonged administration of NMDA antagonist and safener drug to alter neuropathic pain condition
InactiveUS20050148673A1Reduce neurotoxic side effectInherent activityBiocideOrganic active ingredientsNR1 NMDA receptorSide effect
A drug that inhibits NMDA receptors (such as ketamine, a surgical anesthetic) is continuously administered to patients suffering from neuropathic pain. Unless the NMDA antagonist drug has inherent safening activity, this treatment requires a “safener” drug to prevent the neurotoxic side effects of NMDA antagonists. One class of safener drugs that increase the efficacy of the treatment include alpha-2 adrenergic agonists, such as clonidine. The treatment lasts for several days and nights, continuously. A maximum tolerated dosage is titered for each patient, such as by observing slurring of speech, and the patient does not lose consciousness except during normal sleep. Magnesium and / or drugs that inhibit ketamine-degrading enzymes can also be used. Patients who suffered for years from chronic intractable pain emerged from this treatment with apparently permanent relief, or with lasting reductions in their levels of pain.
Owner:HARBUT RONALD E +2
Compositions and methods for the treatment of anorectal disorders
Compositions and methods for the treatment of anorectal disorders are provided in which certain combinations of NO donors, PDE inhibitors, superoxide (O2−) scavengers, β-adrenergic agonists, cAMP-dependent protein kinase activators, α1-adrenergic antagonists, L-type Ca2+ channel blockers, estrogens, ATP-sensitive K+ channel activators and smooth muscle relaxants are used.
Owner:STREHKEHN INT LTD
Carbazolyl-substituted ethanolamines as selective beta -3 agonists
InactiveUS6140352AAvoid insufficient purityBiocideNervous disorderAdrenergic receptor agonistsEthanolamines
PCT No. PCT / US97 / 15230 Sec. 371 Date May 4, 1998 Sec. 102(e) Date May 4, 1998 PCT Filed Aug. 28, 1997 PCT Pub. No. WO98 / 09625 PCT Pub. Date Mar. 12, 1998Disclosed herein are selective beta 3 adrenergic agonists represented by the following structural formula: The variables in the structural formula shown above are defined in the specification. Also disclosed are methods of using these compounds for agonizing the beta 3 adrenergic receptor in patients in need of such treatment, for example, patients in need of treatment for obesity or Type II diabetes.
Owner:ELI LILLY & CO
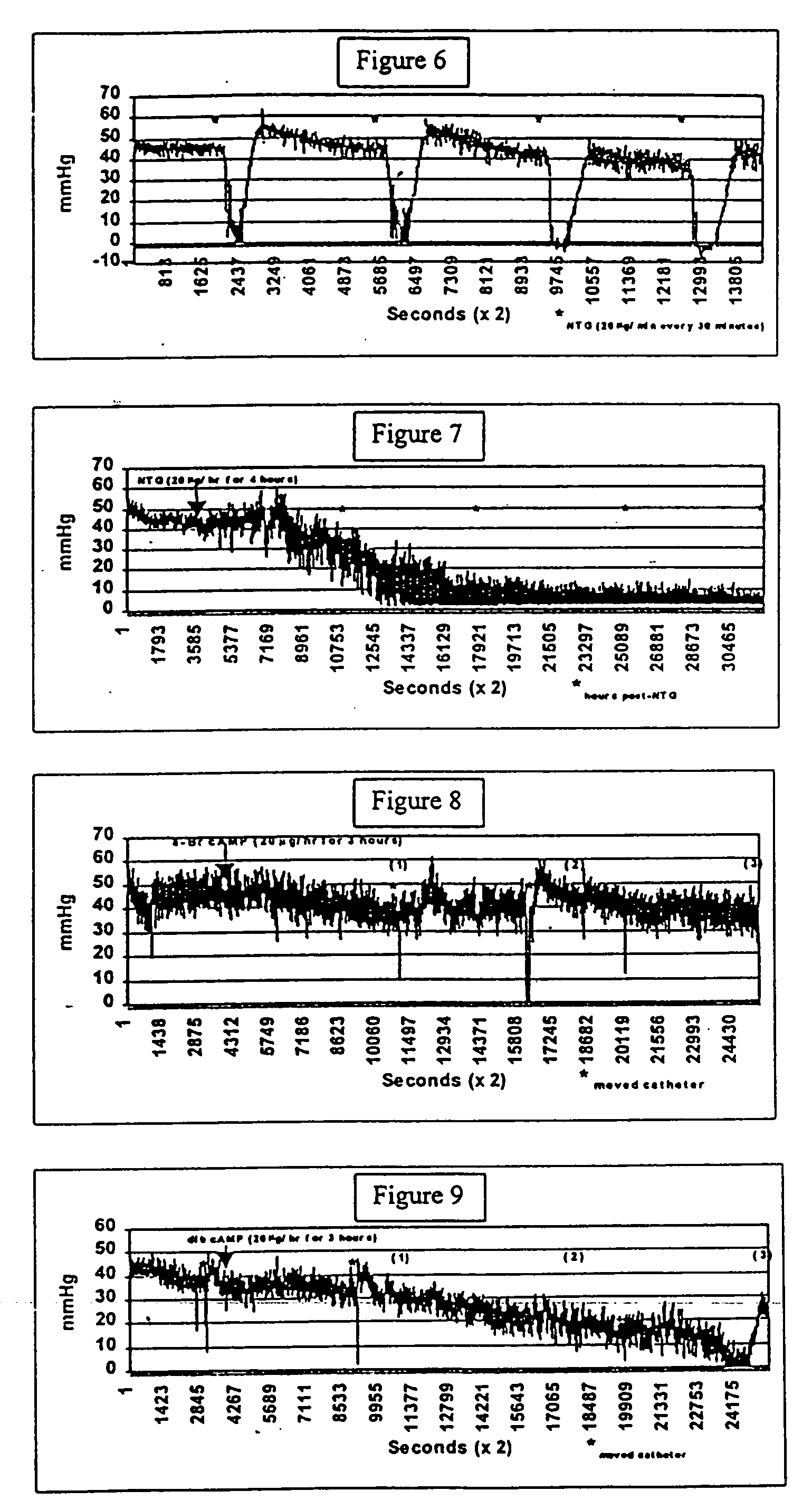
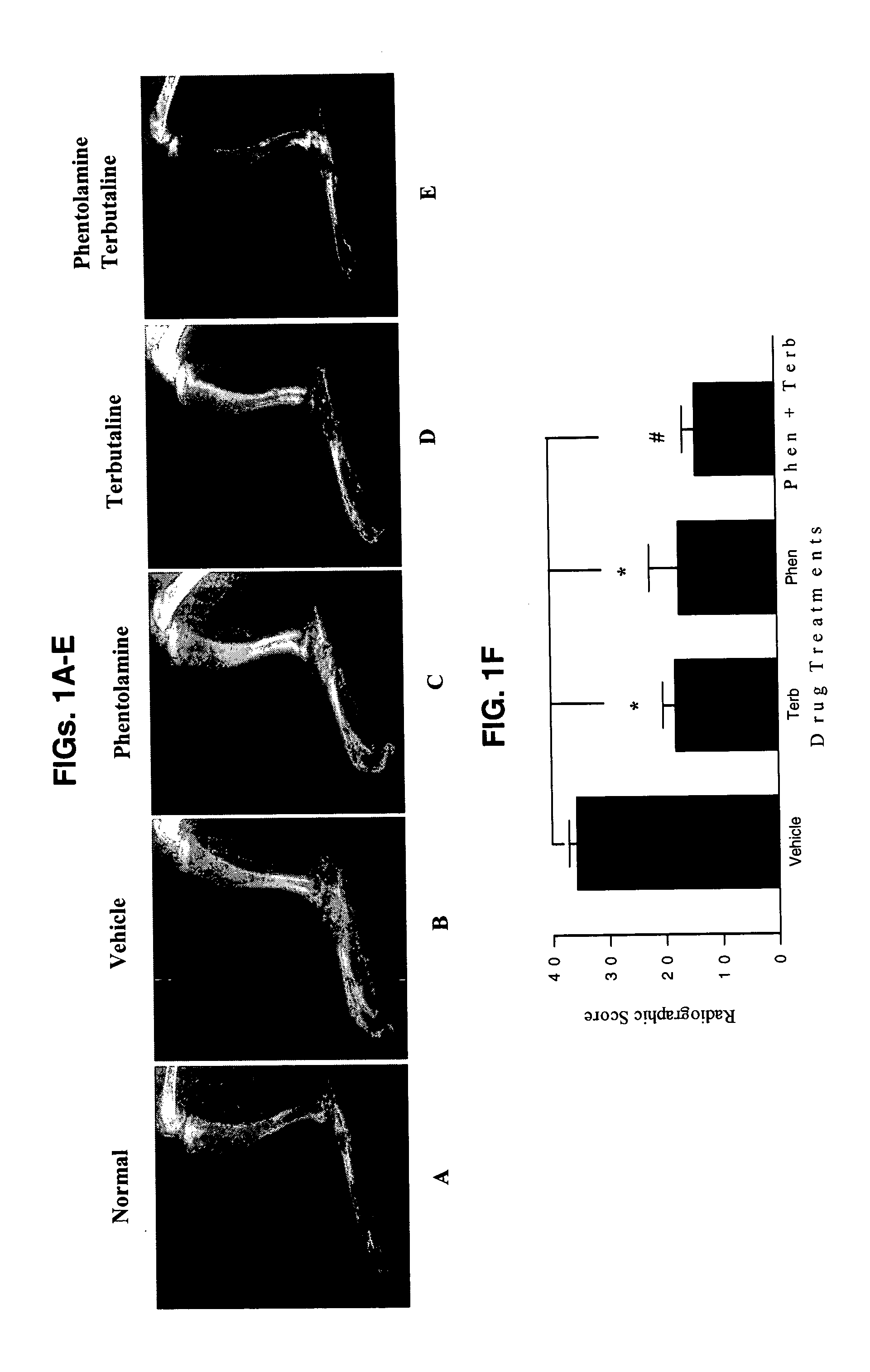
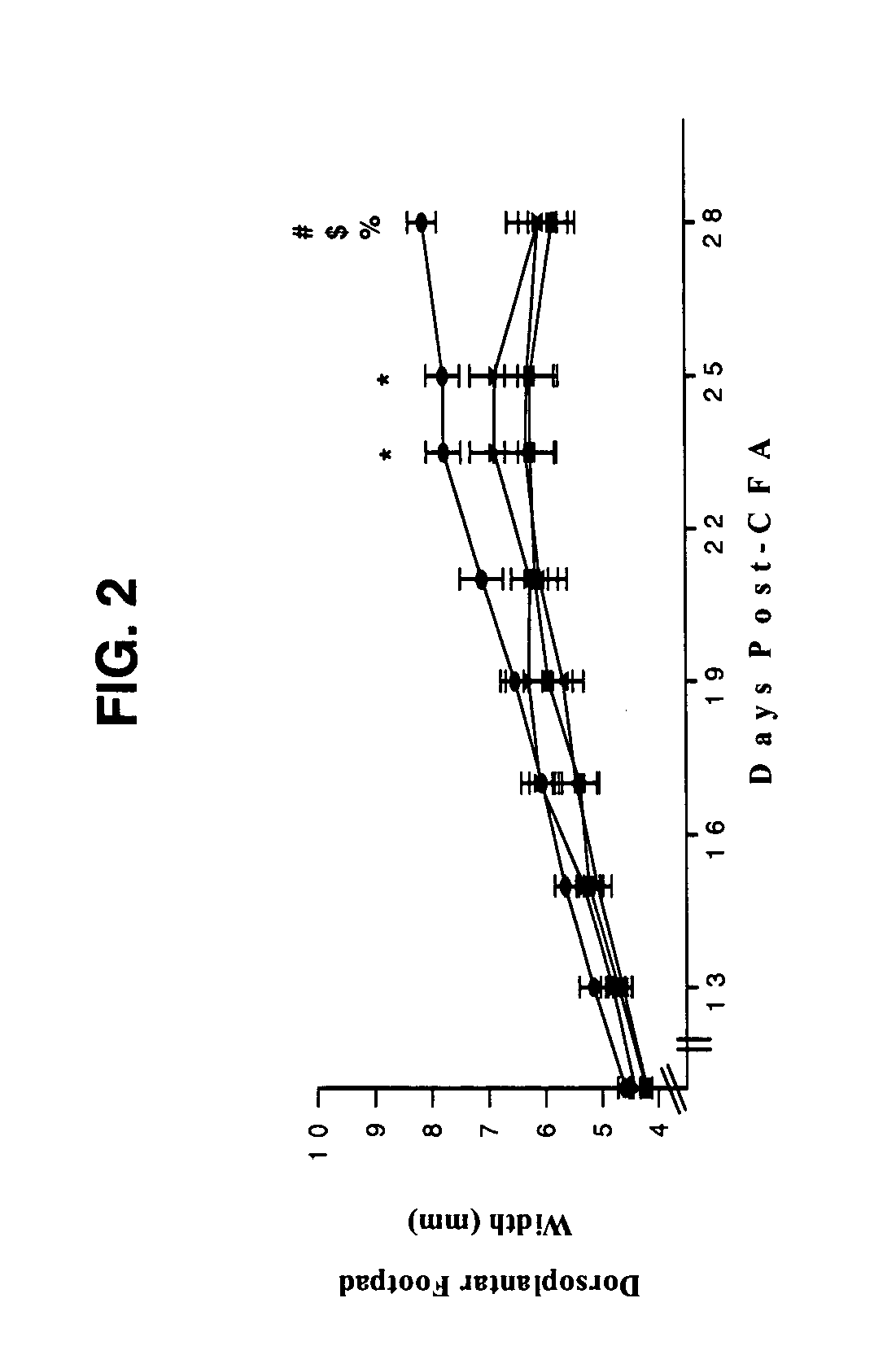
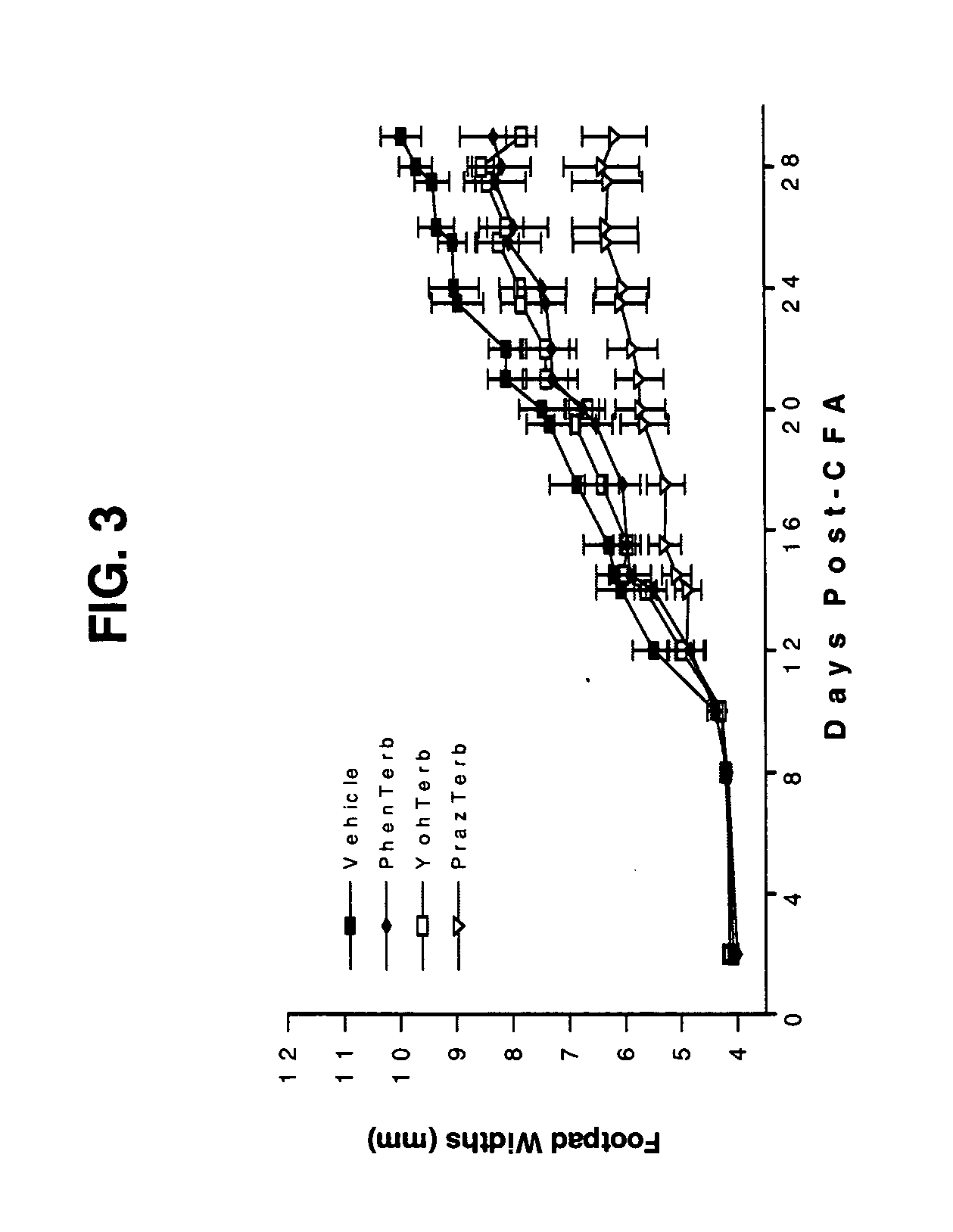
Treatment of inflammatory autoimmune diseases with alpha-adrenergic antagonists and beta-adrenergic agonists
The present invention discloses a novel compound and method for the treatment of inflammatory autoimmune diseases, for example, rheumatoid arthritis, using α-adrenergic antagonists and β-adrenergic agonists in combination. Treatment of animals, namely humans, with an α-adrenergic antagonist, preferably, phentolamine, and a β-adrenergic agonist, preferably terbutaline, in combination can significantly suppress the joint destruction and inflammation due to disease in these animals.
Owner:SUN HEALTH RES INST
Combinations comprising antimuscarinic agents and beta-adrenergic agonists
Combinations comprising (a) a β2 agonist and (b) an antagonist of M3 muscarinic receptors which is 3(R)-(2-hydroxy-2,2-dithien-2-ylacetoxy)-1-(3-phenoxypropyl)-1-azoniabicyclo[2.2.2]octane, in the form of a salt having an anion X, which is a pharmaceutically acceptable anion of a mono or polyvalent acid are useful, e.g., for the treatment of respiratory disease, e.g., asthma or chronic obstructive pulmonary disease.
Owner:ALMIRALL
Methods for treating lower urinary tract disorders using alpha2delta subunit calcium channel modulators with smooth muscle modulators
A method is provided for using α2δ subunit calcium channel modulators or other compounds that interact with the α2δ calcium channel subunit in combination with one or more compounds with smooth muscle modulatory effects to treat and / or alleviate the symptoms associated with painful and non-painful lower urinary tract disorders in normal and spinal cord injured patients. According to the present invention, α2δ subunit calcium channel modulators include GABA analogs (e.g. gabapentin and pregabalin), fused bicyclic or tricyclic amino acid analogs of gabapentin, and amino acid compounds. Compounds with smooth muscle modulatory effects include antimuscarinics, β3 adrenergic agonists, spasmolytics, neurokinin receptor antagonists, bradykinin receptor antagonists, and nitric oxide donors.
Owner:EDUSA PHARMA
.beta.3 adrenergic agonists
InactiveUS6730792B2Reduce the possibilityBiocidePowder deliveryAdrenergic receptor agonistsEnergy expenditure
The present invention relates to a .beta..sub.3 adrenergic receptor agonist of formula (I) or a pharmaceutical salt thereof, which is capable of increasing lipolysis and energy expenditure in cells and, therefore, is useful for treating Type II diabetes and / or obesity. The compound can also be used to lower triglyceride levels and cholesterol levels or raise high density lipoprotein levels or to decrease gut motility. In addition, the compound can be used to reduced neurogenic inflammation or as an antidepressant agent. Compositions and methods for the use of the compounds in the treatment of diabetes and obesity and for lowering triglyceride levels and cholesterol levels or raising high density lipoprotein levels or for decreasing gut motility are also disclosed.
Owner:ELANCO US INC
Delivery of a sympatholytic cardiovascular agent to the central nervous system
A sympatholytic cardiovascular agent delivered by a drug delivery pump to a central nervous system site to alleviate symptoms of acute or chronic cardiac insult or impaired cardiac performance. The drug delivery pump can be external or implantable infusion pump (IIP) coupled with a drug infusion catheter extending to the site. A patient activator can command delivery of a dosage and / or an implantable heart monitor (IHM) coupled with a sensor can detect physiologic parameters associated with cardiac insult or impaired cardiac performance and trigger dosage delivery. The IIP and IHM can be combined into a single implantable medical device (IMD) or can constitute separate IMDs that communicate by any of known communication mechanisms. The sympatholytic cardiovascular agent is one of the group consisting of an alpha-adrenergic agonist and an alpha2-adrenergic agonist (e.g., clonidine, p-aminoclonidine, guanabenz, lidamidine, tizanidine, moxonidine, methyldopa, xylazine, guanfacine, detomidine, medetomidine, and dexmedetomidine).
Owner:MEDTRONIC INC
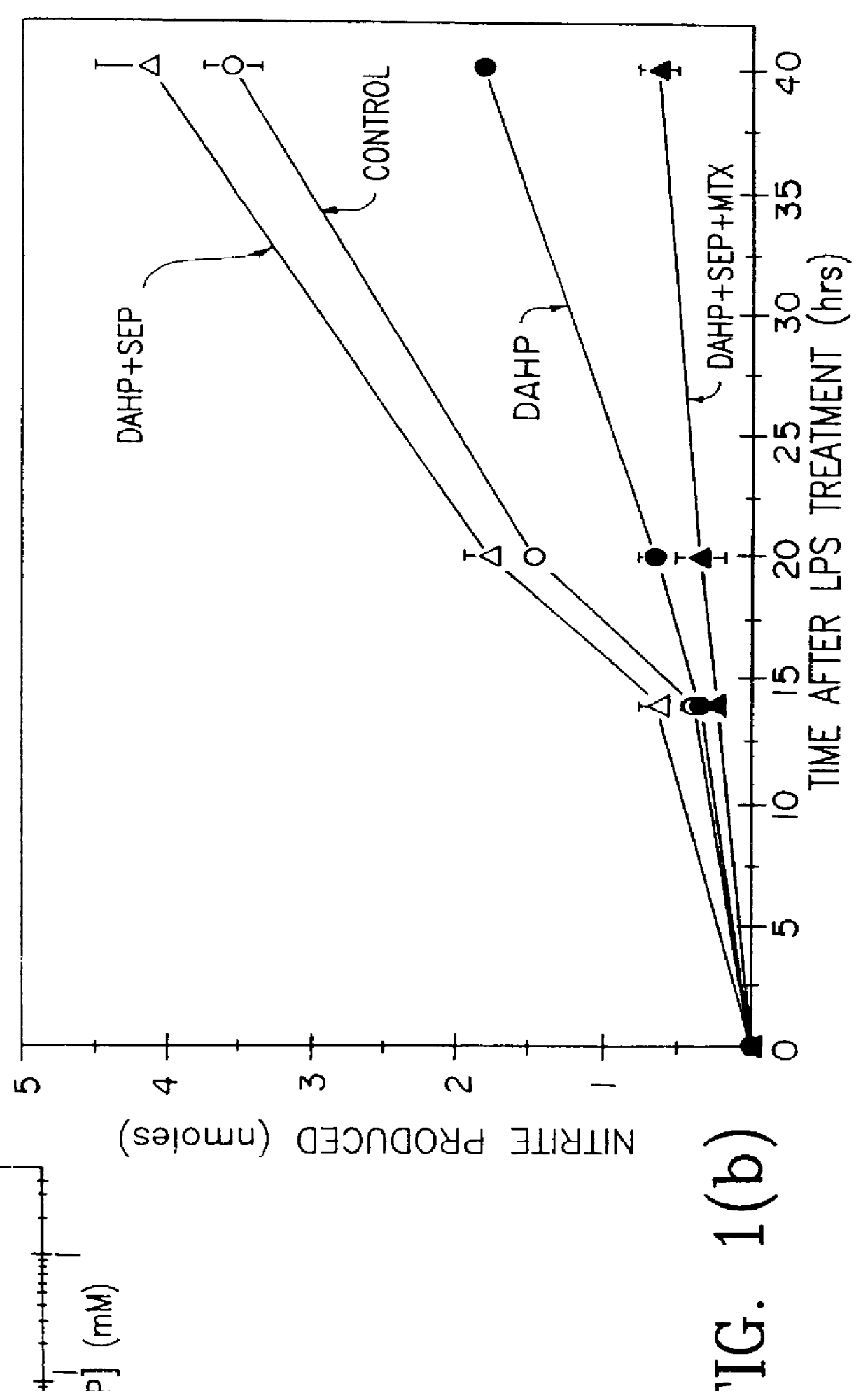
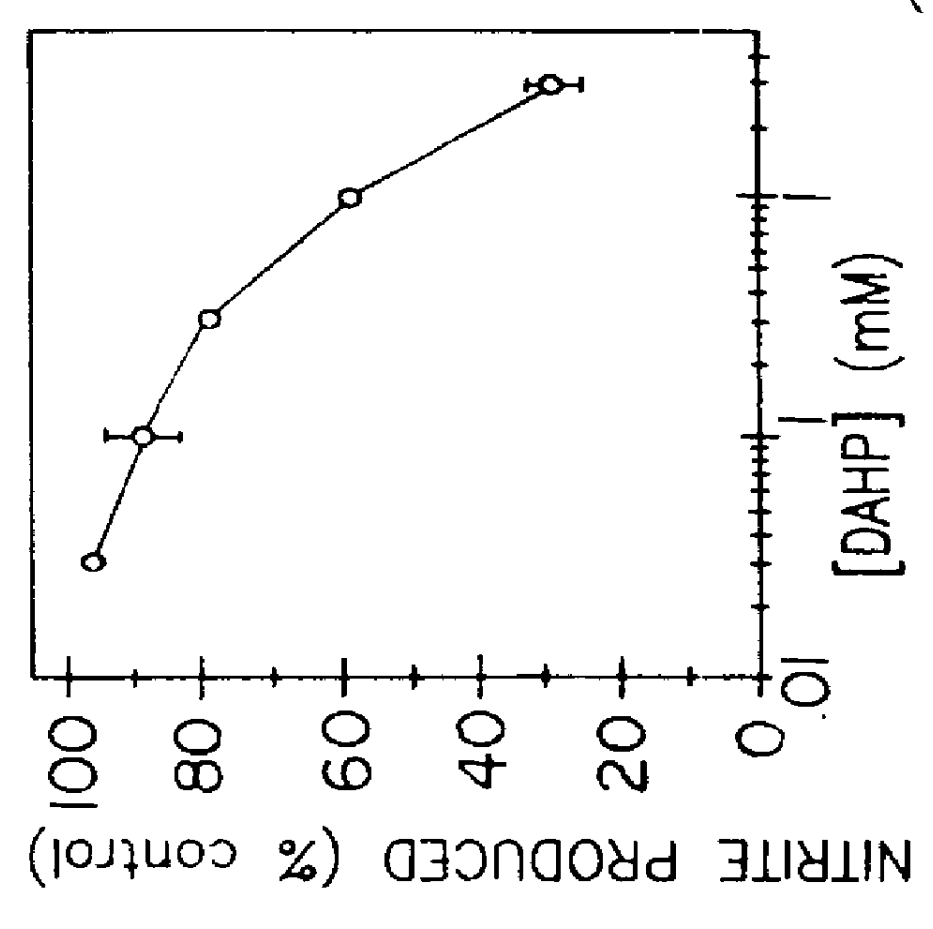
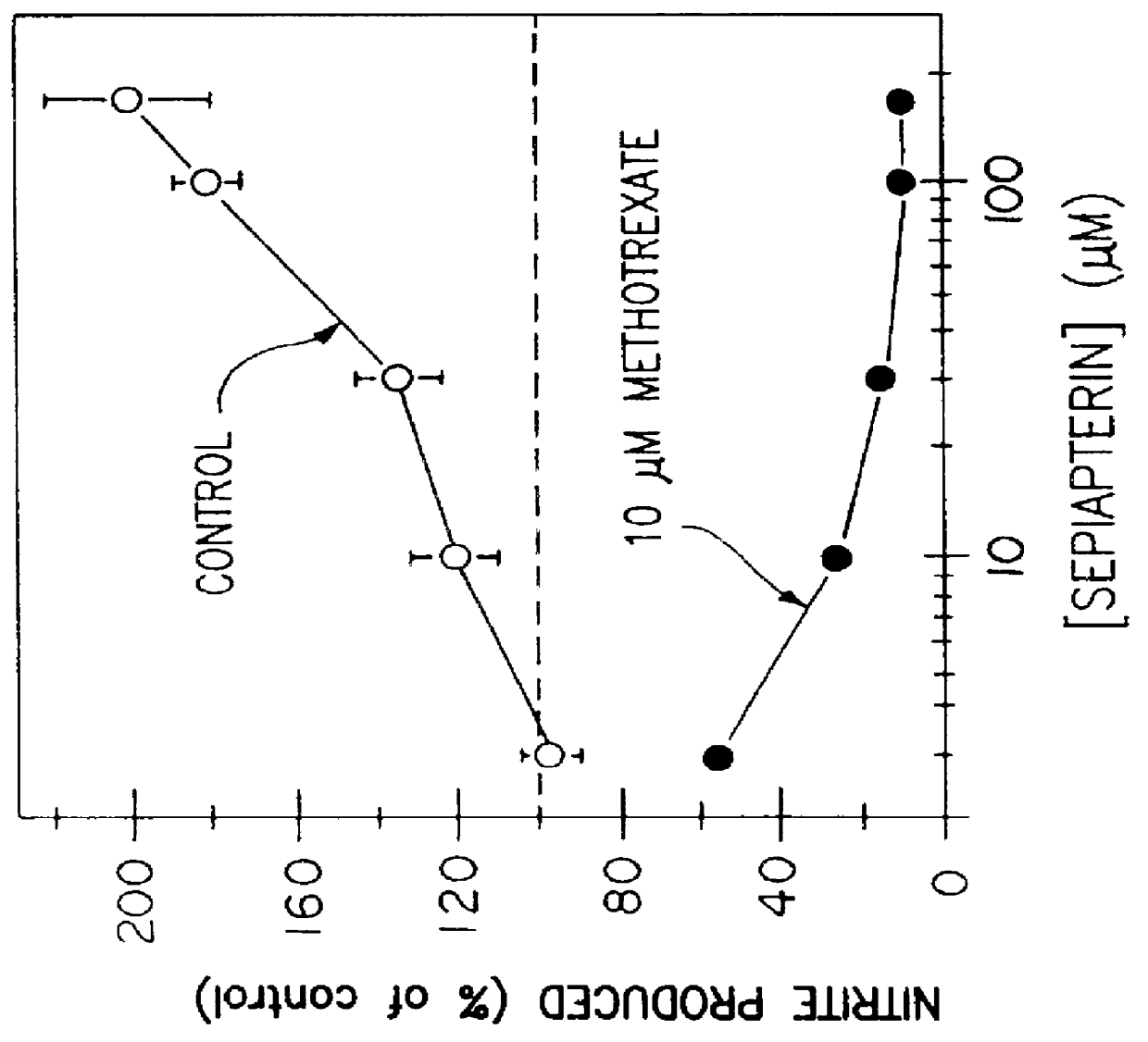
Blocking induction of tetrahydrobioterin to block induction of nitric oxide synthesis
InactiveUS6153615AReduce inhibitionRestore sensitivityBiocidePeptide/protein ingredientsSide effectTreatment effect
Guanosine triphosphate pathway tetrahydrobiopterin synthesis antagonist and / or pterin salvage pathway tetrahydrobiopterin synthesis antagonists are administered to inhibit nitric oxide synthesis from arginine in vascular cells in a subject in need of such inhibition (e.g., for prophylactic or curative effect for endotoxin- or cytokine-induced hypotension or for restoration of vascular contractile sensitivity to pressor agents in the treatment of such hypotension). The tetrahydrobiopterin synthesis antagonist may be administered with alpha 1-adrenergic agonist or with nitric oxide synthase inhibitor. The tetrahydrobiopterin synthesis antagonists are also administered to attenuate inflammation caused by induced nitric oxide production in immune cells. Unwanted counterproductive or side effects can be eliminated or ameliorated by administration additionally of levodopa with or without carbidopa and L-5-hydroxytryptophane.
Owner:CORNELL RES FOUNDATION INC
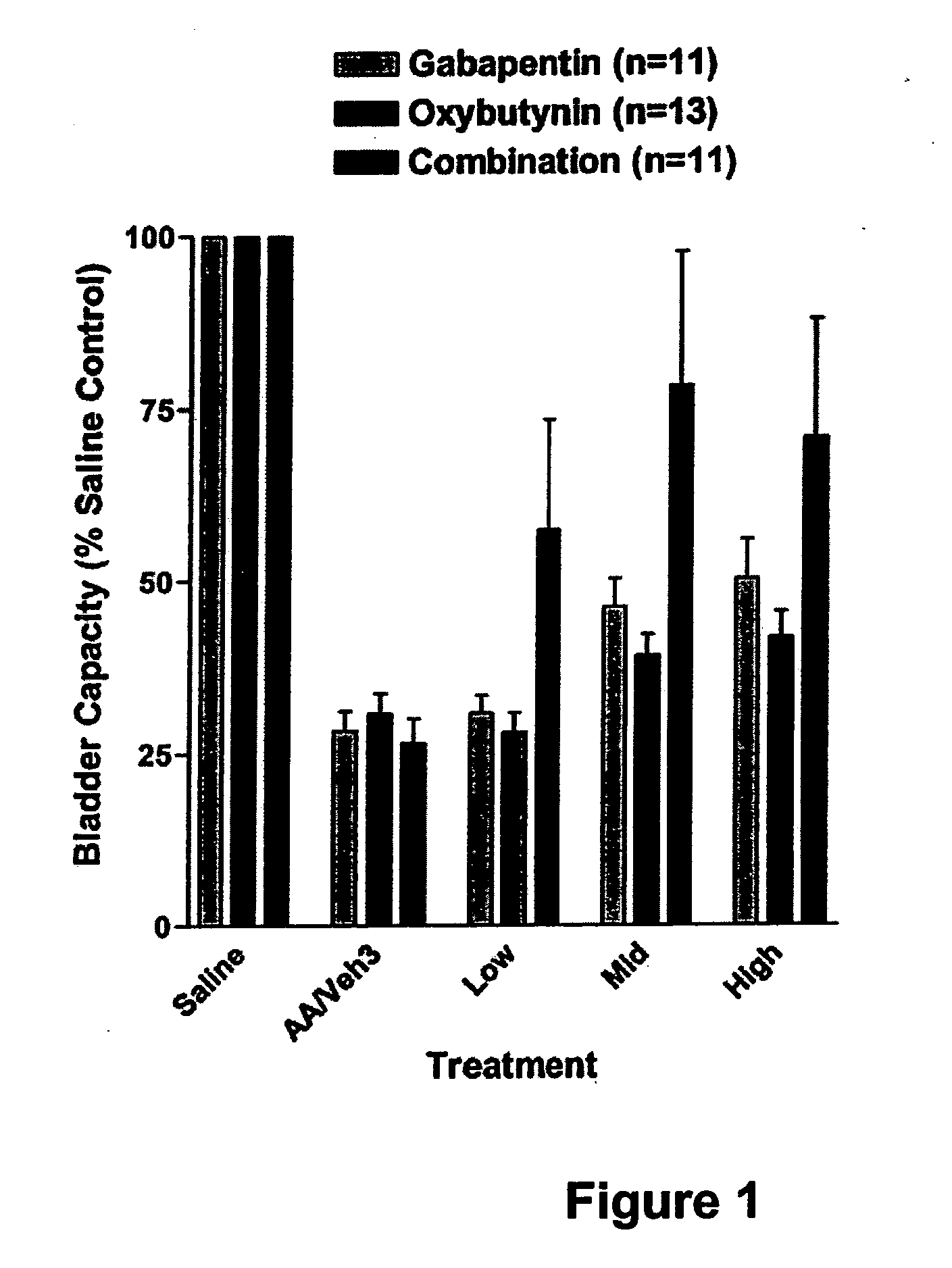
Methods for decreasing detrusor muscle overactivity
InactiveUS20050239890A1Limited efficacyReduce patient complianceBiocideOrganic active ingredientsDiseaseGabapentin
A method is provided for using α2δ subunit calcium channel modulators or other compounds that interact with the α2δ calcium channel subunit in combination with one or more compounds with smooth muscle modulatory effects to treat and / or alleviate the symptoms associated with painful and non-painful lower urinary tract disorders in normal and spinal cord injured patients. According to the present invention, α2δ subunit calcium channel modulators include GABA analogs, e.g., gabapentin and pregabalin, fused bicyclic or tricyclic amino acid analogs of gabapentin, and amino acid compounds. Compounds with smooth muscle modulatory effects include antimuscarinics, β3 adrenergic agonists, spasmolytics, neurokinin receptor antagonists, bradykinin receptor antagonists, and nitric oxide donors.
Owner:DYNOGEN PHARM INC
Extended release alpha-2 agonist pharmaceutical dosage forms
InactiveUS20050118256A1Sufficient effectFacilitated releaseBiocideDigestive systemAdrenergic receptor agonistsAgonist drugs
Disclosed is an extended release pharmaceutical formulation containing at least an alpha-2 adrenergic agonist, such as tizanidine, for the treatment and prevention of spasticity in a subject, e.g., painful inflammatory conditions associated with skeletal muscle spasms.
Owner:GLENMARK PHARMACEUTICALS LIMITED
Selective beta3 adrenergic agonists
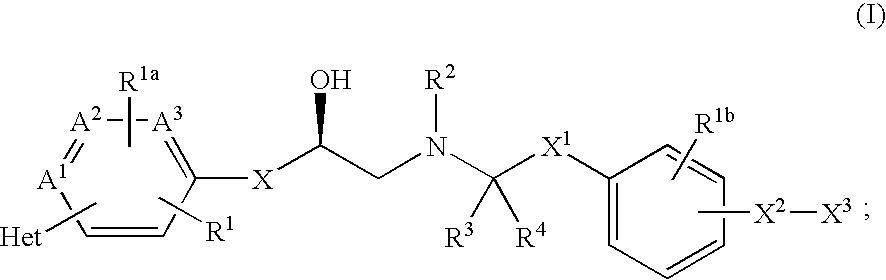
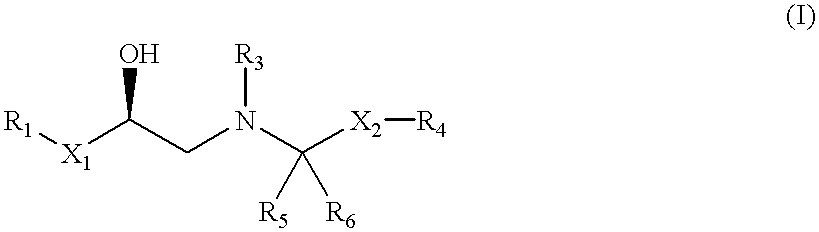
The present invention is in the field of medicine, particularly in the treatment of Type II diabetes and obesity. More specifically, the present invention relates to selective beta3 adrenergic receptor agonists useful in the treatment of Type II diabetes and obesity. The invention provides compounds and method of treating type II diabetes, comprising administering to a mammal in need thereof compounds of the Formulas I and II:
Owner:ELI LILLY & CO
Methods for treating lower urinary tract disorders using alpha2delta subunit calcium channel modulators with smooth muscle modulators
InactiveUS20060247311A1Need lessLimited efficacyBiocidePeptide/protein ingredientsDiseaseGabapentinoid
A method is provided for using α2δ subunit calcium channel modulators or other compounds that interact with the α2δ calcium channel subunit in combination with one or more compounds with smooth muscle modulatory effects to treat and / or alleviate the symptoms associated with painful and non-painful lower urinary tract disorders in normal and spinal cord injured patients. According to the present invention, α2δ subunit calcium channel modulators include GABA analogs (e.g. gabapentin and pregabalin), fused bicyclic or tricyclic amino acid analogs of gabapentin, and amino acid compounds. Compounds with smooth muscle modulatory effects include antimuscarinics, β3 adrenergic agonists, spasmolytics, neurokinin receptor antagonists, bradykinin receptor antagonists, and nitric oxide donors.
Owner:DYNOGEN PHARM INC
Microneedle devices and methods
A medical device, comprising: an array of microneedles, and a coating disposed on the microneedles, wherein the coating comprises: a local anesthetic selected from the group consisting of lidocaine, prilocaine, and a combination thereof; and a local anesthetic dose-extending component selected from the group consisting of alpha 1 adrenergic agonists, alpha 2 adrenergic agonists, and a combination thereof; wherein the local anesthetic is present in an amount of at least 1 wt-% based upon total weight of solids in the coating, and wherein the dose-extending component / local anesthetic weight ratio is at least 0.0001; a medical device, comprising an array of dissolvable microneedles, the microneedles comprising: a dissolvable matrix material; at least 1 wt-% of a local anesthetic selected from the group consisting of lidocaine, prilocaine, and a combination thereof; and a local anesthetic dose-extending component selected from the group consisting of alpha 1 adrenergic agonists, alpha 2 adrenergic agonists, and a combination thereof; wherein the dose-extending component / local anesthetic weight ratio is at least 0.0001, and wherein wt-% is based upon total weight of solids in all portions of the dissolvable microneedles which contain the local anesthetic; a method of extending a topically delivered local anesthetic dose in mammalian tissue using the devices; and methods of making the devices are provided.
Owner:3M INNOVATIVE PROPERTIES CO
Ophthalmic formulation and method for ameliorating presbyopia
An ophthalmic formulation having an effective amount of a parasympathomimetic agent comprising pilocarpine, or a pharmaceutically acceptable salt thereof, and one or more α1 adrenergic agonists or antagonists is disclosed. The ophthalmic formulation may enable treatment of conditions adversely affecting the visual acuity of a patient, including presbyopia. A method of using the disclosed ophthalmic formulation to treat or ameliorate symptoms of presbyopia is also disclosed.
Owner:VEJARANO RESTREPO LUIS FELIPE
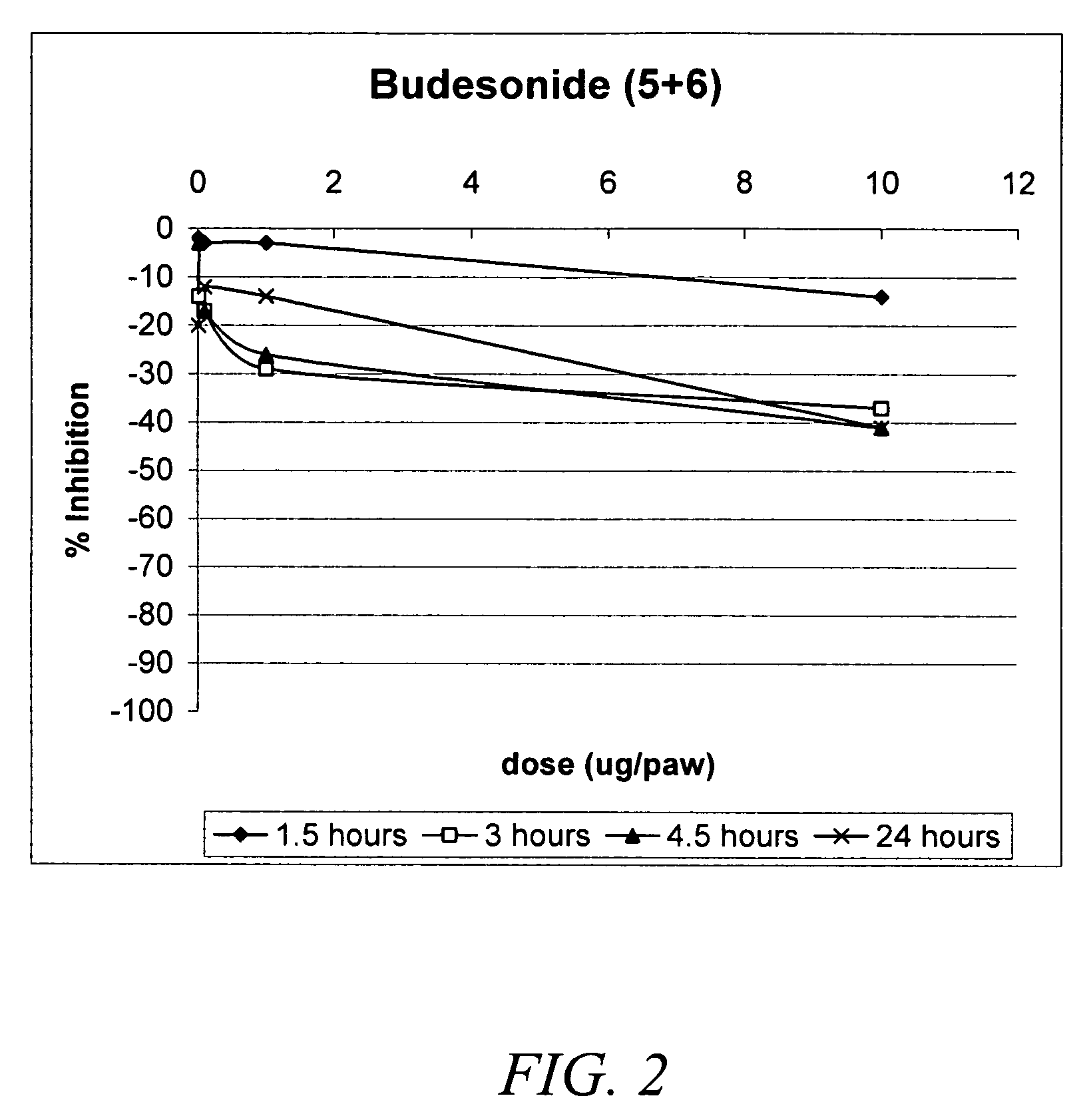
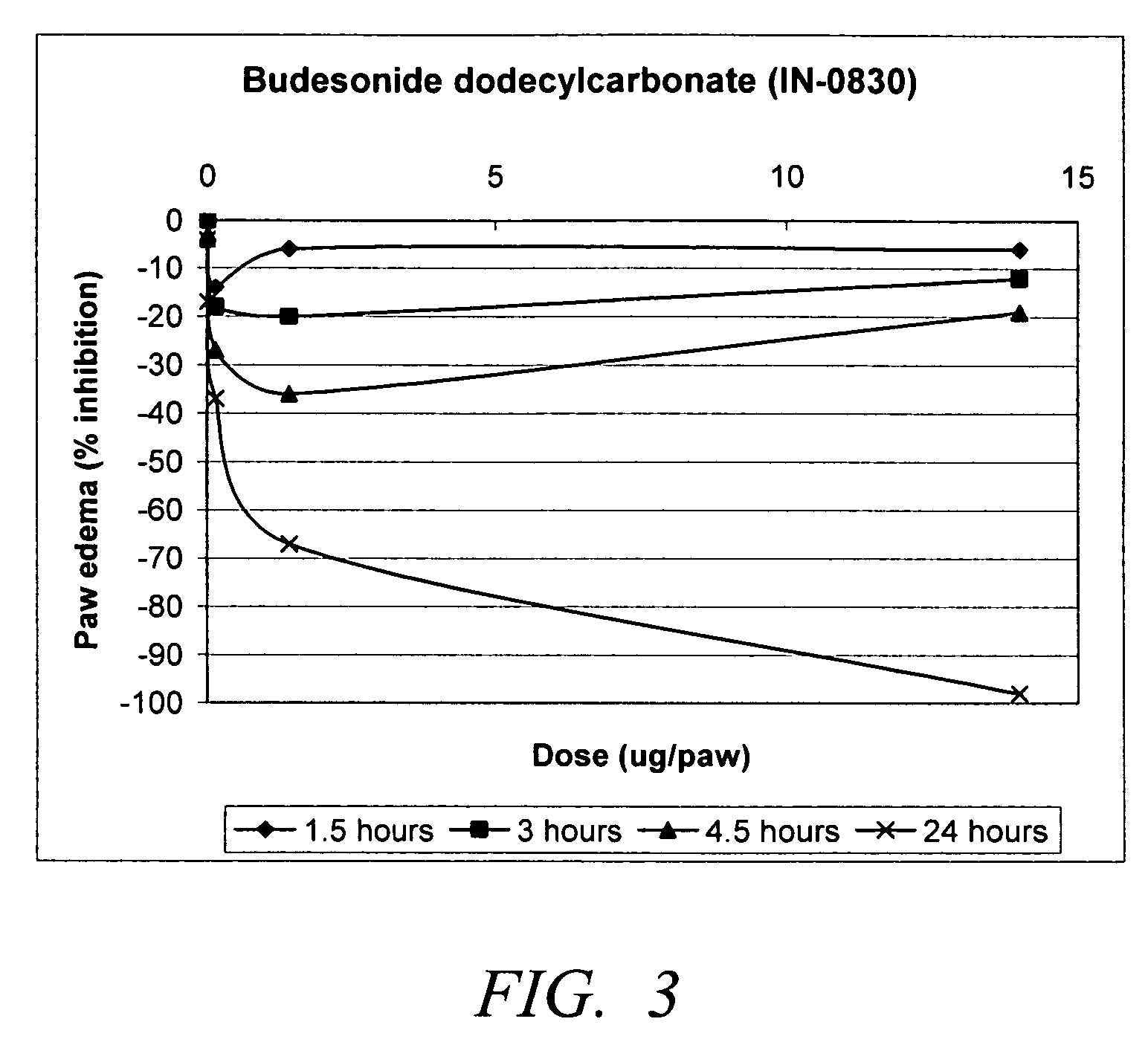
Carbonate and carbamate modified forms of glucocorticoids in combination with B2 adrenergic agonists
Compositions containing β2 adrenergic agonists in combination with carbonates and carbamates of the formulaand in combination with related steroid carbonates and carbamates are disclosed. The compositions are useful for treating bronchospasm, for inducing bronchodilation and for treating rhinitis, asthma, and chronic obstructive pulmonary disease (COPD) and inflammatory diseases, particularly by inhalation.
Owner:SUNOVION PHARMA INC
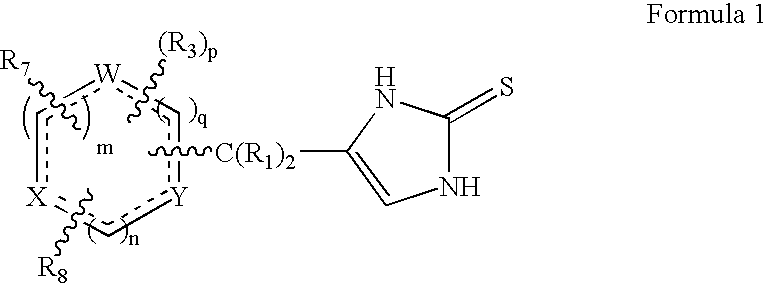
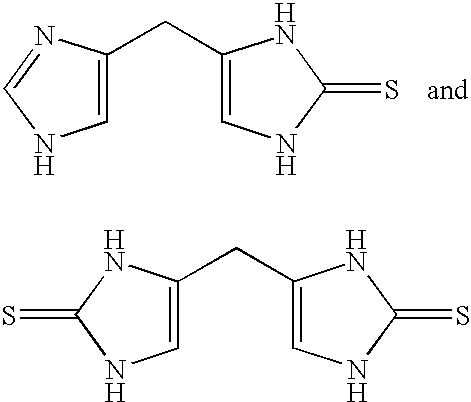
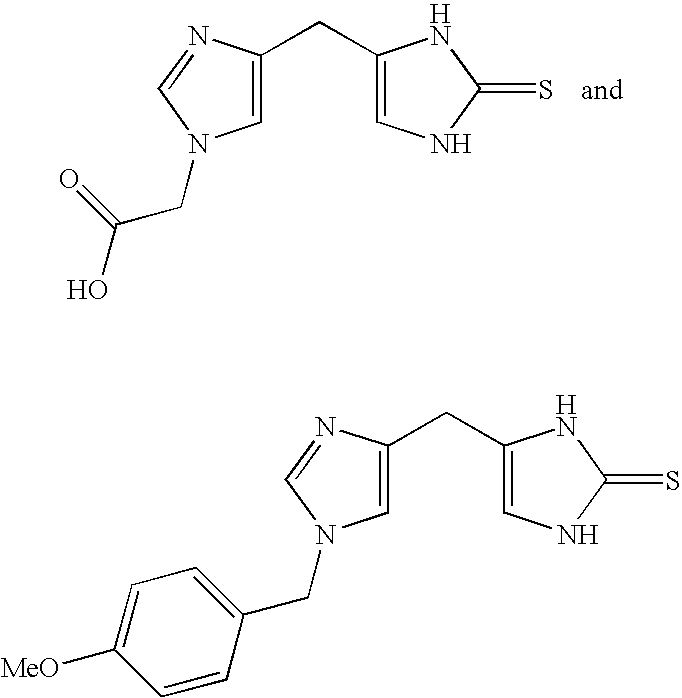
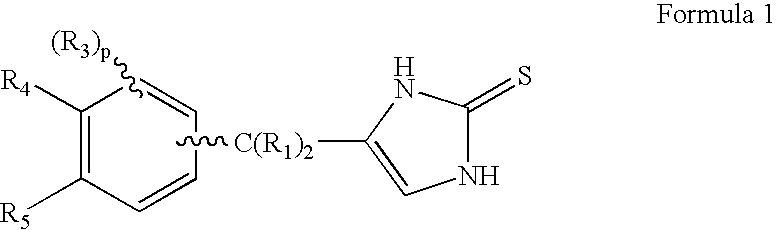
4-(Heteroaryl-methyl and substituted heteroaryl-methyl)-imidazole-2-thiones acting as alpha2 adrenergic agonists
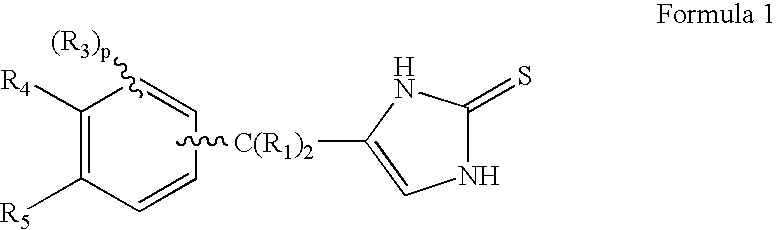
InactiveUS20060069143A1Antibacterial agentsOrganic active ingredientsDiseaseAlpha2 adrenergic agonist
Compounds of Formula 1 where the variables have the meaning defined in the specification are agonists of alpha2 adrenergic receptors. Several compounds of the disclosure are specific or selective to alpha2B and / or alpha2C adrenergic receptors in preference over alpha2A adrenergic receptors. Additionally some of the claimed compounds have no or only minimal cardivascular and / or sedatory activity. The compounds of Formula 1 are useful as medicaments in mammals, including humans, for treatment of diseases and or alleviations of conditions which are responsive to treatment by agonists of alpha2 adrenergic receptors. Compounds of Formula 1 which have no significant cardiovascular and / or sedatory activity are useful for treating pain and other conditions with minimal side effects.
Owner:ALLERGAN INC
Method of Treatment Using Alpha-1-Adrenergic Agonist Compounds
ActiveUS20100113377A1Prevents increase amountIncrease the areaBiocideSugar derivativesAdrenergic receptor agonistsΑ adrenergic
Methods for treating or preventing cardiomyopathy in a subject by administering an al adrenergic receptor agonist, wherein the treatment does not result in increased blood pressure are provided.
Owner:RGT UNIV OF CALIFORNIA
Compositions and methods for prevention and treatment of cachexia
InactiveUS20070149465A1Prevent and treat cachexiaLower Level RequirementsBiocideMetabolism disorderAdrenergic receptor agonistsPharmacology
Compositions and methods for preventing and treating wasting disorders, such as cachexia and anorexia, are provided. In one aspect, the present invention provides a method for preventing and treating a wasting disorder in a mammal. In one embodiment, the method of the invention comprises administering to such mammal a macrolide and a β2-adrenergic agonist in combination such that the macrolide and said β2-agonist are administered in amounts effective to prevent or at least alleviate said wasting disorder.
Owner:THE RELEEF INITIATIVE
Methods and compositions for modulating the mobilization of stem cells
InactiveUS7939057B2Enhance mobilizationPrevent egressBiocidePeptide/protein ingredientsDiseaseAdrenergic receptor agonists
Methods and compositions for modulating the mobilization of stem cells, particularly for promoting or increasing the mobilization of hematopoietic stem cells from the bone marrow to the peripheral blood are disclosed. In particular, the invention relates to the use of adrenergic agonists that act in concert with a mobilization compound or agent. The mobilization agent(s) may act to decrease the expression or function of the chemokine, CXCL12, or may act to block or antagonize CXCR4. The invention also relates to methods of using these compounds or agents for enhancing the mobilization of hematopoietic stem cells when harvesting of the stem cells is necessary for the treatment of diseases, disabilities or conditions whereby transplantation of such cells would be beneficial in ameliorating the symptoms associated with such diseases, disabilities or conditions. Methods of screening for novel agents and pharmaceutical compositions comprising these agents are also disclosed.
Owner:MT SINAI SCHOOL OF MEDICINE
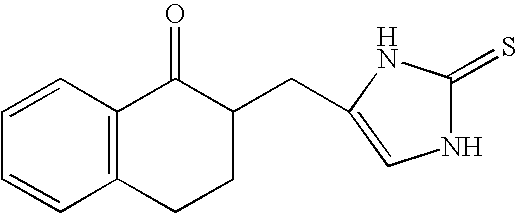
4-(Condensed cyclicmethyl)-imidazole-2-thiones acting as alpha2 adrenergic agonists
InactiveUS20060069144A1Minimal cardiovascularMinimal sedatory activityBiocideSenses disorderDiseaseSide effect
Compounds of Formula 1 where the variables have the meaning defined in the specification are agonists of alpha2 adrenergic receptors. Several compounds of the disclosure are specific or selective to alpha2B and / or alpha2C adrenergic receptors in preference over alpha2A adrenergic receptors. Additionally some of the claimed compounds have no or only minimal cardivascular and / or sedatory activity. The compounds of Formula 1 are useful as medicaments in mammals, including humans, for treatment of diseases and or alleviations of conditions which are responsive to treatment by agonists of alpha2 adrenergic receptors. Compounds of Formula 1 which have no significant cardiovascular and / or sedatory activity are useful for treating pain and other conditions with minimal side effects.
Owner:ALLERGAN INC
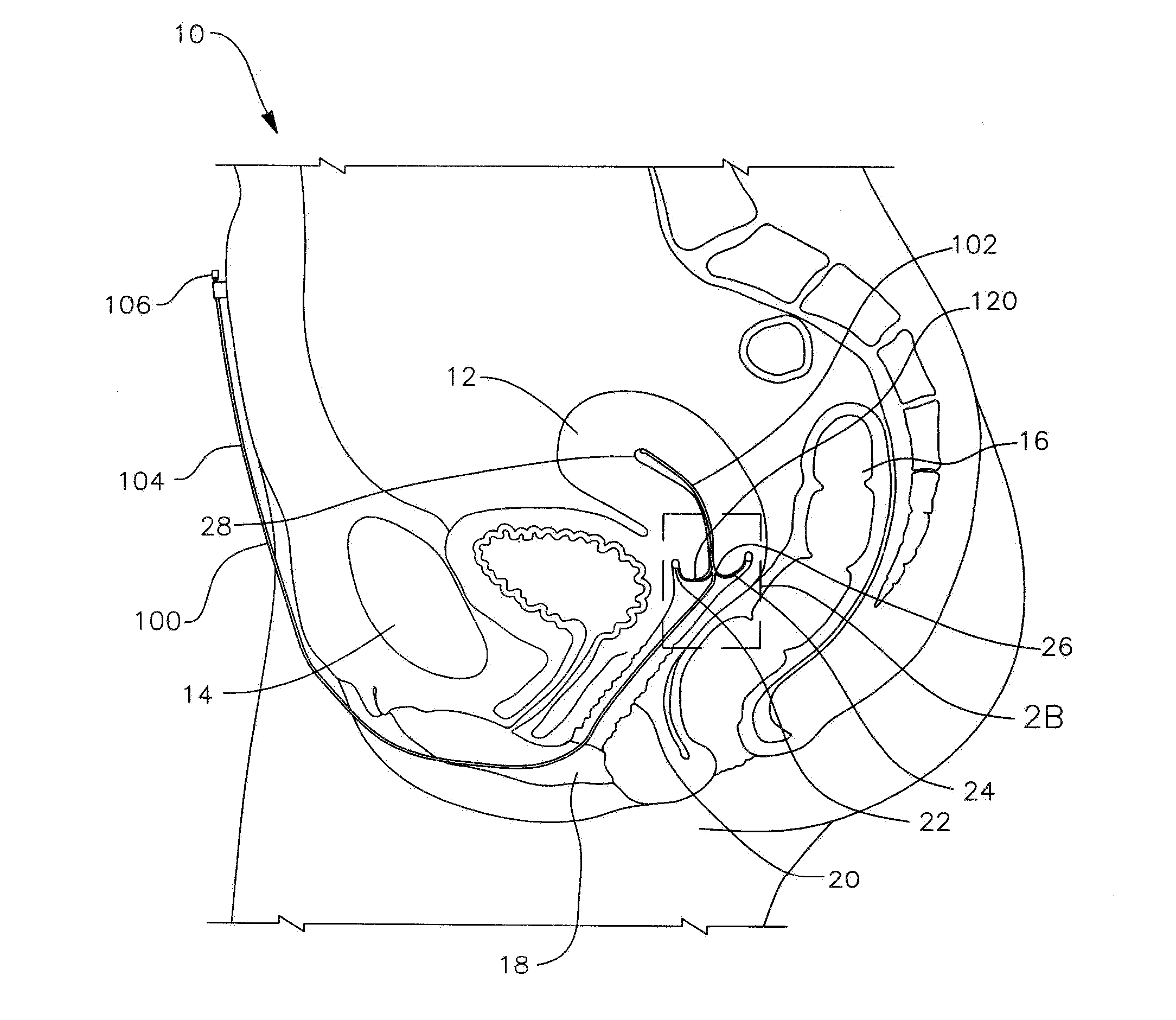
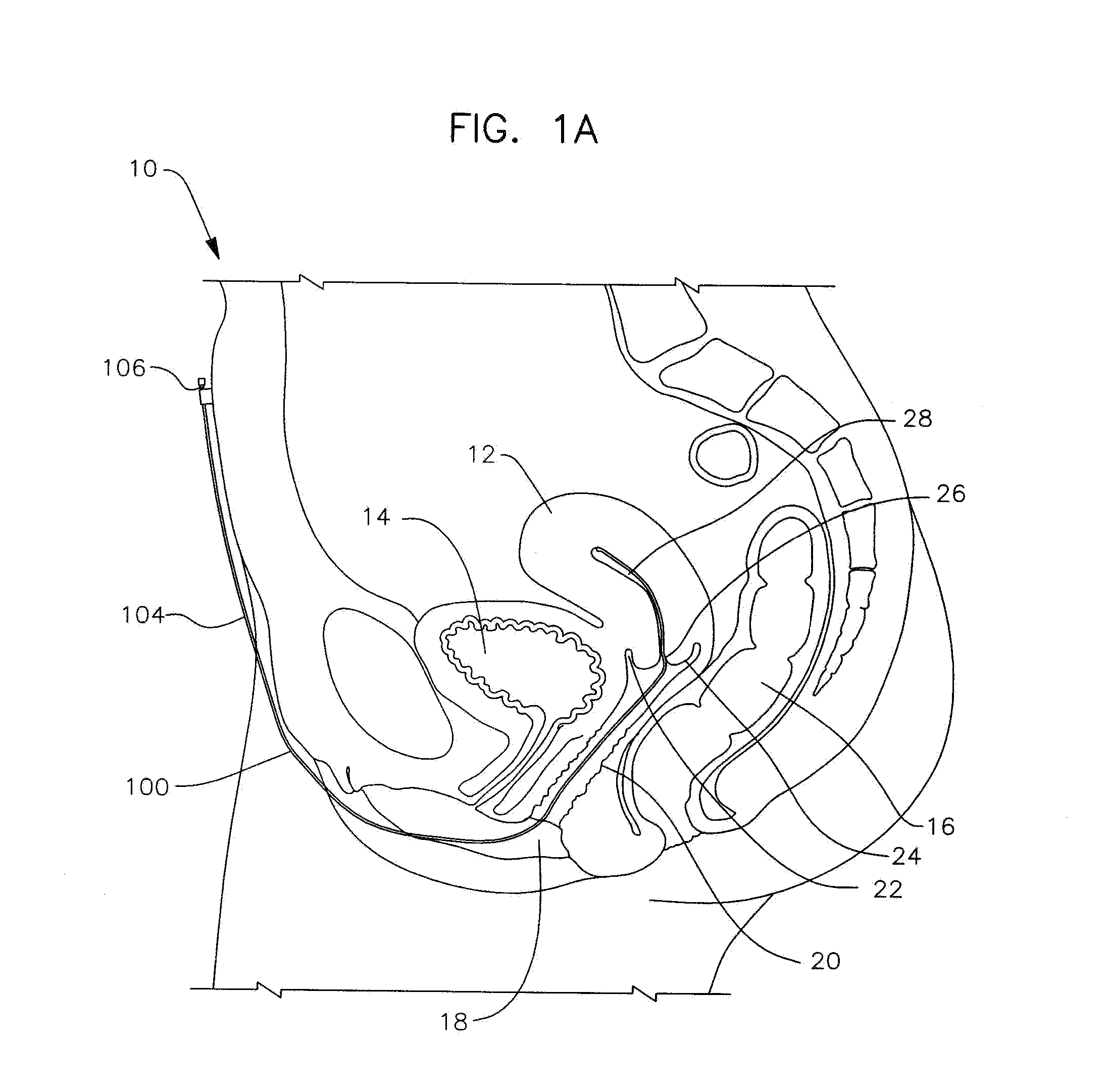
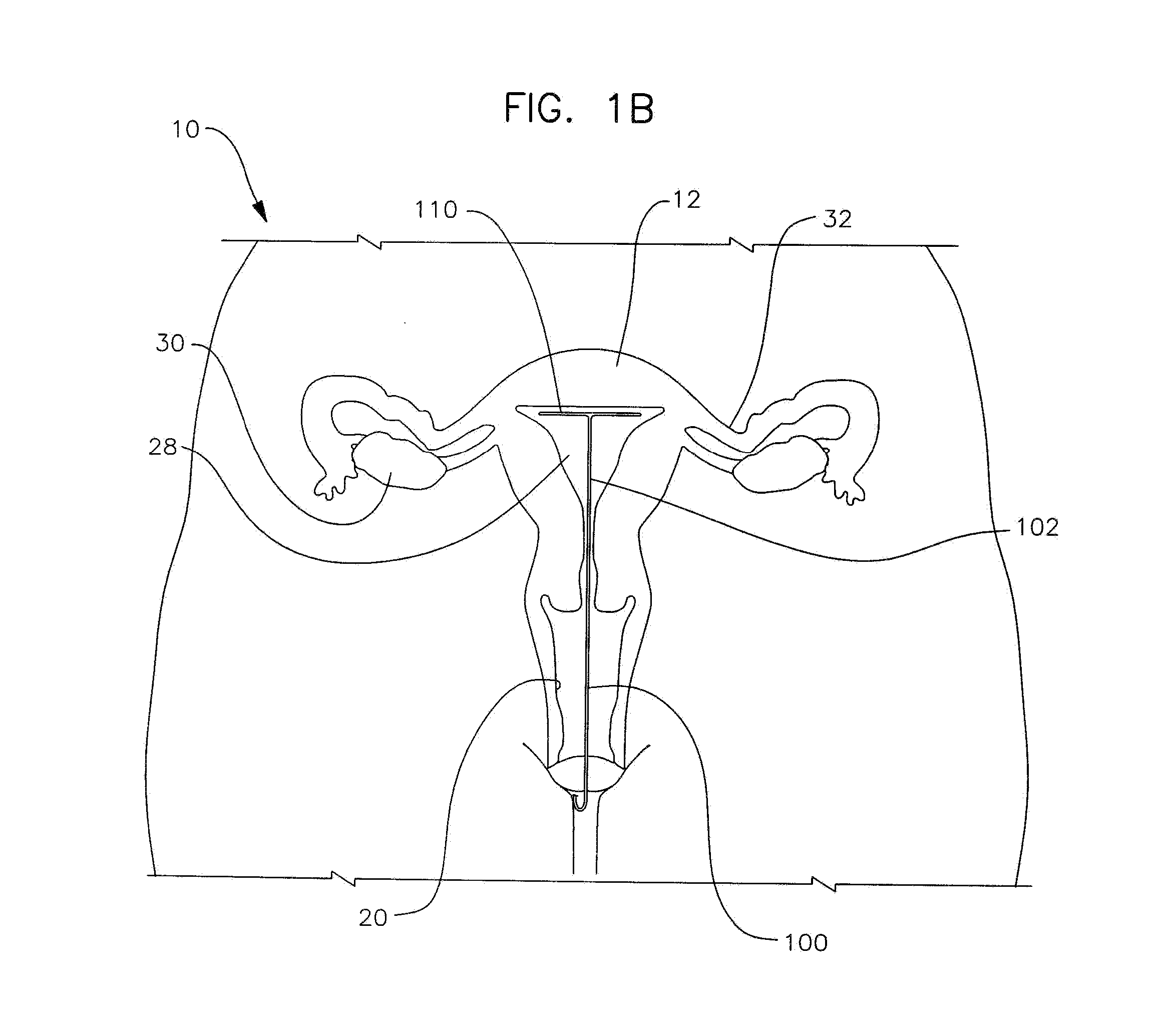
Apparatus and method for the treatment of abnormal uterine bleeding
InactiveUS20130150418A1Regulated release rateExtended maintenance periodBiocideOrganic active ingredientsSurgical operationBleeding postpartum
Method and apparatus are disclosed for applying a therapeutic amount of a non-systemic vasoconstrictor inside the uterus to control abnormal uterine bleeding. The abnormal bleeding can be due to excessive menstrual blood flow, bleeding from a surgical procedure, postpartum bleeding or any other acute or chronic condition. The vasoconstrictor includes topical agents such as an alpha-adrenergic agonist, for example oxymetazoline. The delivery system can include a catheter having means for retaining position of a distal portion within the uterus. A proximal portion can extend outside of the body for coupling to a vasoconstrictor source, or alternatively, the proximal portion can terminate within the vaginal canal and include a docking port for coupling to a source of vasoconstrictor that is inserted therein. In other embodiments, an applicator is disclosed that is positioned in fluid communication with the lumen of the cervix and allows application of a vasoconstrictor therein.
Owner:GOEDEKE STEVEN D +3
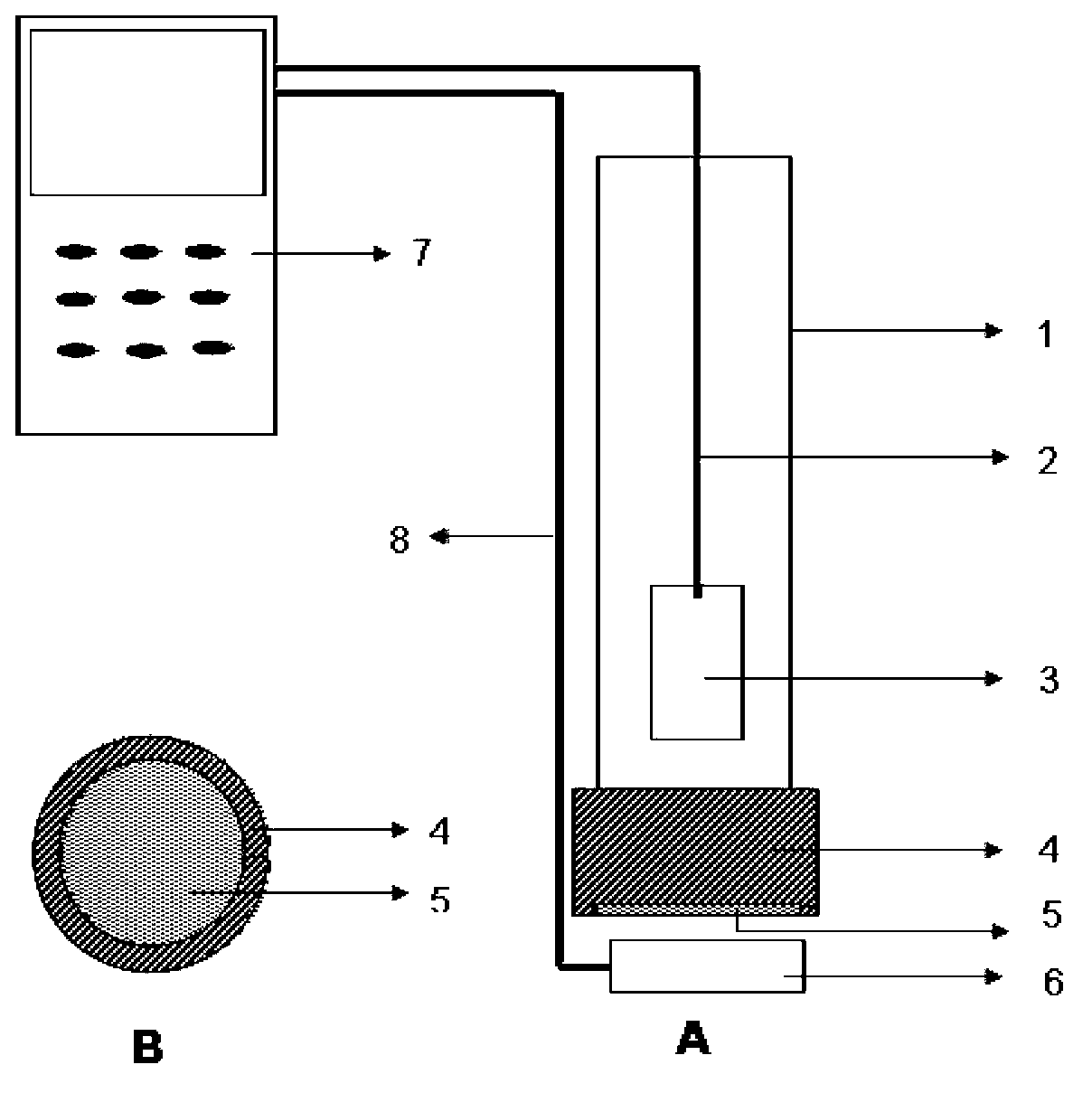
Potentiometric sensing electrode for testing adrenergic agonist and sensor thereof
InactiveCN103076377ASimple structureEasy to fixMaterial electrochemical variablesPlatinumAdrenergic receptor agonists
The invention discloses a potentiometric sensing electrode for testing an adrenergic agonist and a sensor thereof. The sensing electrode comprises an electrode cable, a platinum electrode, an insulating sleeve, an electrode cap, a molecular imprinting membrane and a potential electrode solution; the platinum electrode is arranged in the insulating sleeve, and the electrode cable is drawn out from the upper end portion of the insulating sleeve; an end cap of the electrode cap is in an annular hollow structure, and an inner circle area on the inner side of the end cap is covered with the molecular imprinting membrane provided with a molecular imprinting pore structure; the outer side on the lower end portion of the insulating sleeve is closely sleeved with the electrode cap to fix the molecular imprinting membrane between the electrode cap and the insulating sleeve; and the potential electrode solution immersing at least the lower end of the platinum electrode is poured in the insulating sleeve. According to the potentiometric sensing electrode for detecting the adrenergic agonist and the sensor thereof, the potentiometric sensing electrode uses the molecular imprinting membrane with high peculiarity as a receptor of an electrochemical sensor and has the advantages that the test sensitivity is high, the operation is simple, the sensing electrode is convenient to carry, and the like, and field tests of adrenergic agonists are achieved.
Owner:SHANGHAI JIAO TONG UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com