Microneedle devices and methods
A technology of microneedles and microneedle arrays, which is applied in the direction of microneedles, needles, and pharmaceutical formulations, and can solve the problems of expensive system manufacturing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0174] Preparations containing lidocaine and clonidine
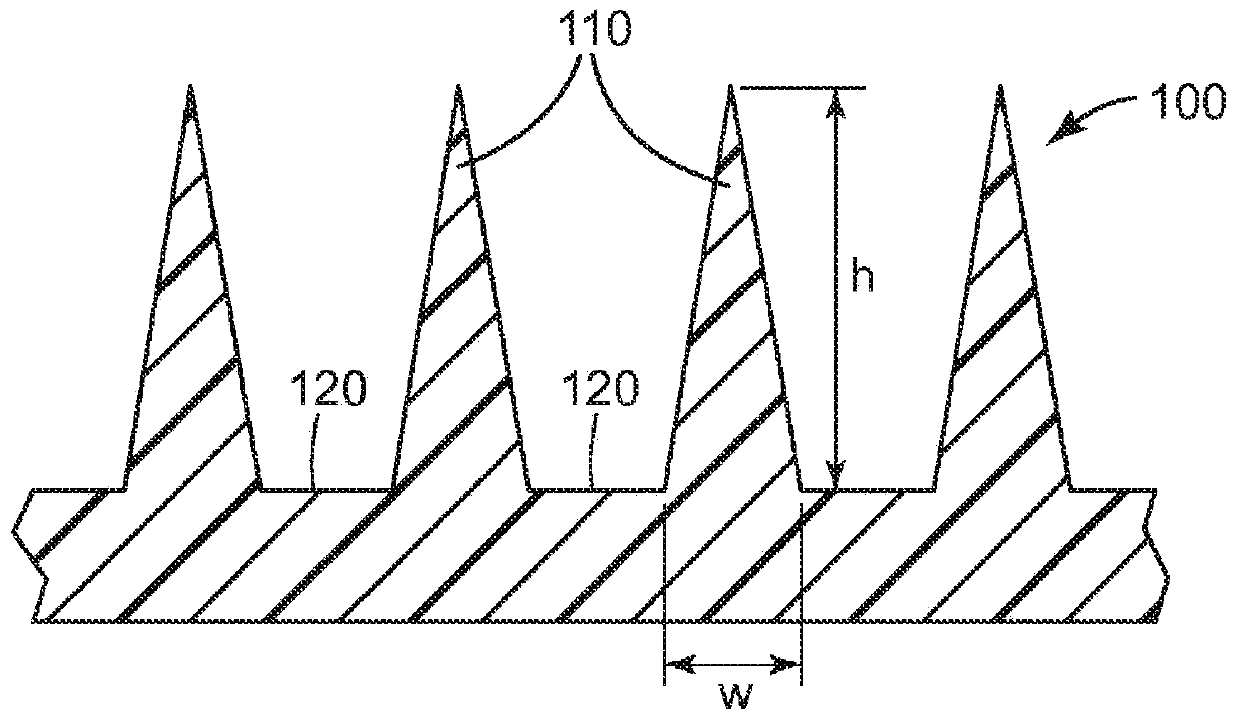
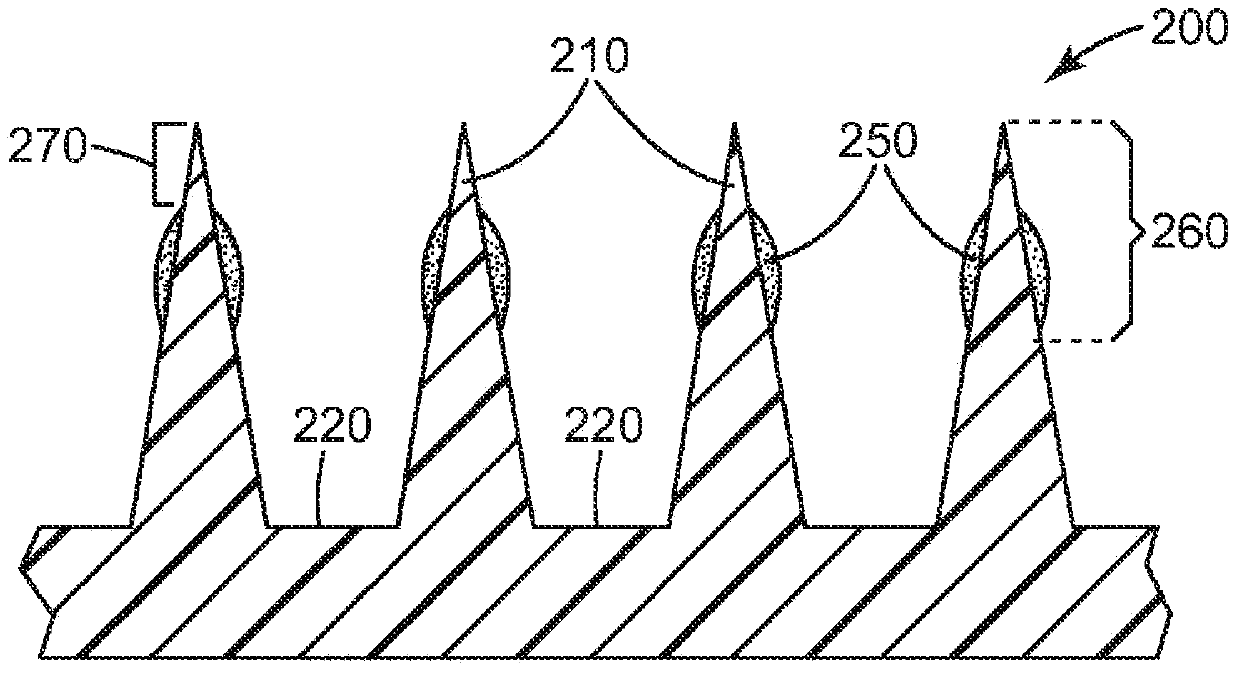
[0175] The microneedle array uses a surface area of about 1.27 cm 2 Class VI medical grade liquid crystal polymer (LCP) ( MT1300 was obtained from Ticona Plastics, Auburn Hills, Michigan, USA and was injection molded (3M Company, St. Paul, MN, USA). Each microneedle array is characterized by 316 quadrangular pyramidal microneedles arranged in an octagonal pattern, where the height of the microneedles is nominally 500 μm, the aspect ratio is about 3:1, and the tips between adjacent microneedles reach The tip distance is nominally 550 microns.
[0176] Using the dip-coating method, a mixture of 30% dextran (Pharmacosmos, Holbaek, Denmark), 30% lidocaine hydrochloride (Sigma-Aldrich, St. Sigma, St.Louis, MO)) and 0.3% clonidine hydrochloride (Spectrum Chemical & Laboratory Products (Spectrum Chemical & Laboratory Products, New Brunswick, NJ) of New Brunswick, New Jersey, U.S.A.) on the microneedle array. Prior to ...
example 2
[0187] Preparations containing lidocaine and epinephrine
[0188] The microneedle array uses a surface area of about 1.27 cm 2 Class VI medical grade liquid crystal polymer (LCP) ( MT1300 was obtained from Ticona Plastics, Auburn Hills, Michigan, USA and was injection molded (3M Company, St. Paul, MN, USA). Each microneedle array is characterized by 316 quadrangular pyramidal microneedles arranged in an octagonal pattern, where the height of the microneedles is nominally 500 μm, the aspect ratio is about 3:1, and the tips between adjacent microneedles reach The tip distance is nominally 550 microns.
[0189] A mixture of 30% dextran (Pharmacosmos, Holbaek, Denmark), 30% lidocaine hydrochloride (Sigma-Aldrich, St. Louis, MO, USA) was used by dip coating. Sigma, St.Louis, MO)) and 0.03% epinephrine tartrate (Sigma, St.Louis, MO, USA, Sigma, St.Louis, MO) to apply lidocaine to the microneedle array superior. Prior to coating, microneedle arrays were cleaned with 70% iso...
example 3
[0200] Preparations containing prilocaine and clonidine
[0201] The microneedle array uses a surface area of about 1.27 cm 2 Class VI medical grade liquid crystal polymer (LCP) ( MT1300 was obtained from Ticona Plastics, Auburn Hills, Michigan, USA and was injection molded (3M Company, St. Paul, MN, USA). Each microneedle array is characterized by 316 quadrangular pyramidal microneedles arranged in an octagonal pattern, where the height of the microneedles is nominally 500 μm, the aspect ratio is about 3:1, and the tips between adjacent microneedles reach The tip distance is nominally 550 microns.
[0202] A mixture of 30% dextran (from Pharmacosmos, Holbaek, Denmark), 15% prilocaine hydrochloride (Spectrum Chemical & Laboratory, New Brunswick, NJ, USA) was used by dip coating. Products (Spectrum Chemical & Laboratory Products, New Brunswick, NJ)) and 0.15% clonidine hydrochloride (Spectrum Chemical & Laboratory Products (Spectrum Chemical & Laboratory Products, New B...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Height | aaaaa | aaaaa |
| Linear | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com