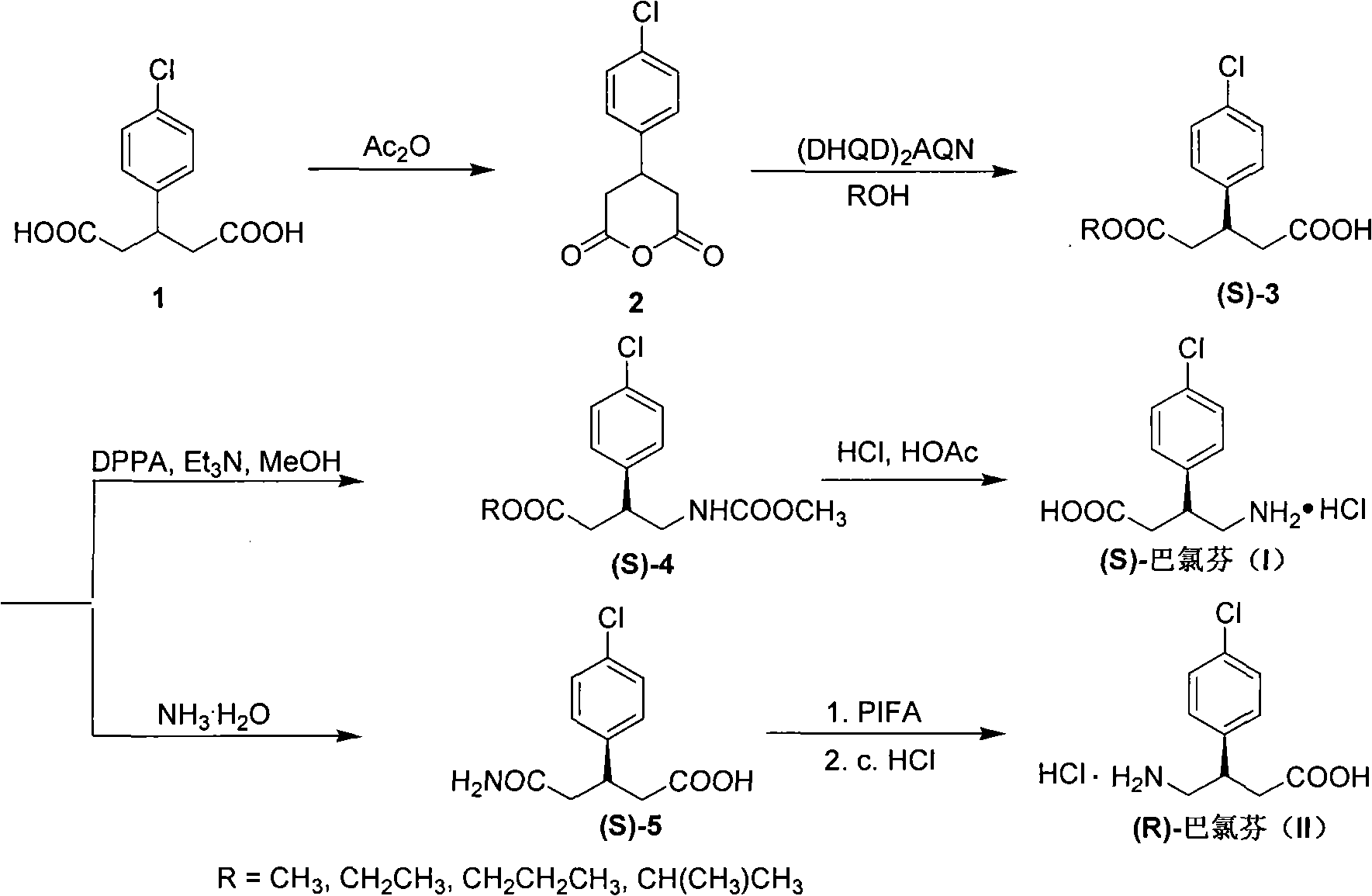
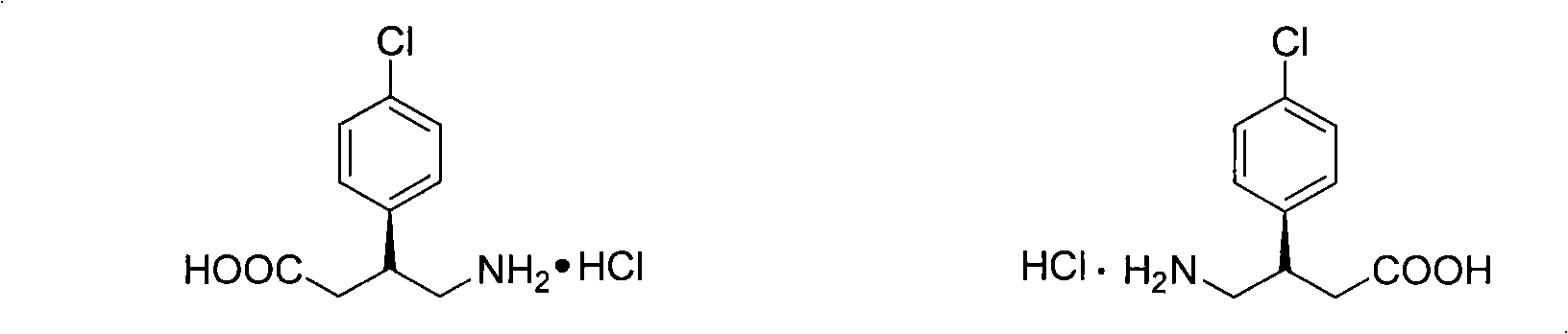Method for preparing chiral baclofen
A baclofen, chiral technology, applied in the field of preparation of chiral baclofen-baclofen,-baclofen), can solve the problems of expensive reagents, complicated operation, long reaction route, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0027] 3-(4-Chlorophenyl)glutaric acid (3.70 g, 15.29 mmol) was added into acetic anhydride (4.2 mL, 45.87 mmol), and refluxed until completely dissolved. Cool to room temperature, add diethyl ether (3 mL) dropwise, filter, wash with a little cold diethyl ether, and dry to obtain 2.77 g of 3-(4-chlorophenyl)glutaric anhydride, yield: 81%, mp: 128-129°C. 1 H NMR (500MHz, CDCl 3 ): δ2.81-2.87(m, 2H), 3.07-3.11(m, 2H), 3.40-3.45(m, 1H), 7.16(d, J=9.0Hz, 2H), 7.37(d, J=9.0 Hz, 2H); 13 C NMR (125MHz, CDCl 3 ): 33.51, 36.91(2C), 127.63(2C), 129.49(2C), 133.97, 137.52, 165.55(2C); FT-IR(KBr, cm -1 ): 1759 (CO); MS (m / z, %rel intensity): 226 (M + , 37 Cl, 6), 224 (M + , 35 Cl, 15), 140(33), 138(100), 115(9), 103(26), 77(9); HRMS(ESI) calcd for C 11 h 10 ClO 3 [M+H] + : 225.0313, found: 225.0316.
example 2
[0029] Under the protection of argon, add 3-(4-chlorophenyl)glutaric anhydride (0.32g, 1.43mmol) into 90mL of anhydrous ether, stir, cool, add (DHQD) 2 AQN (0.39g, 0.45mmol) was added dropwise to anhydrous methanol (0.46g, 14.30mmol) under temperature control at -40°C. After the addition was complete, the reaction was continued at temperature control at -40°C for 120h. Hydrochloric acid (1N, 42 mL) was added, warmed to room temperature, extracted with ethyl acetate (3×100 mL), dried and concentrated. Column chromatography (cyclohexane / ethyl acetate=15:1, then ethyl acetate) gave (S)-3-(4-chlorophenyl)glutaric acid monomethyl ester 0.27g, yield: 75%, 95%ee, mp: 103-104°C; [ α ] D 25 = - 8.0 ( c 0.88 , CHC l 3 ) . ...
example 3
[0031] At room temperature, (S)-3-(4-chlorophenyl)glutaric acid monomethyl ester (0.35g, 1.37mmol) was dissolved in anhydrous benzene (20mL), and diphenylphosphoryl azide (DPPA ) (0.57g, 2.00mmol) and Et 3 N (0.21g, 2.0mmol), reflux reaction for 7h, cooled to room temperature, room temperature reaction overnight. Anhydrous methanol (0.15 g, 4.67 mmol) was added dropwise, and the reaction was refluxed for 10 h. Cool to room temperature, concentrate under reduced pressure, add ethyl acetate, saturated NaHCO 3 washed, washed with water, washed with 5% HCl, washed with water, dried, and concentrated to obtain a crude product. Column chromatography (cyclohexane / ethyl acetate=3:1) gave yellow oil (S)-3-(4-chlorophenyl)-4-methoxycarbonylaminobutyric acid methyl ester 0.24g, yield : 62%. 1 H NMR (500MHz, CDCl 3 ): δ2.46-2.63(m, 2H), 3.20-3.22(m, 2H), 3.38(m, 1H), 3.49(s, 3H), 3.51(s, 3H), 4.70(brs, 1H), 7.04(d, J=8.5Hz, 2H), 7.18(d, J=8.5Hz, 2H); 13 CNMR (125MHz, CDCl 3 ): 38....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com