Solid dispersions containing an apoptosis-inducing agent
a technology of apoptosis and solid dispersions, which is applied in the direction of biocide, drug composition, immunological disorders, etc., can solve the problems of not being able to achieve the effect of improving the clinical utility of an apoptosis-inducing agent, not being able to achieve the effect of apoptosis-inducing agents, and being unable to meet the clinical needs of patients
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
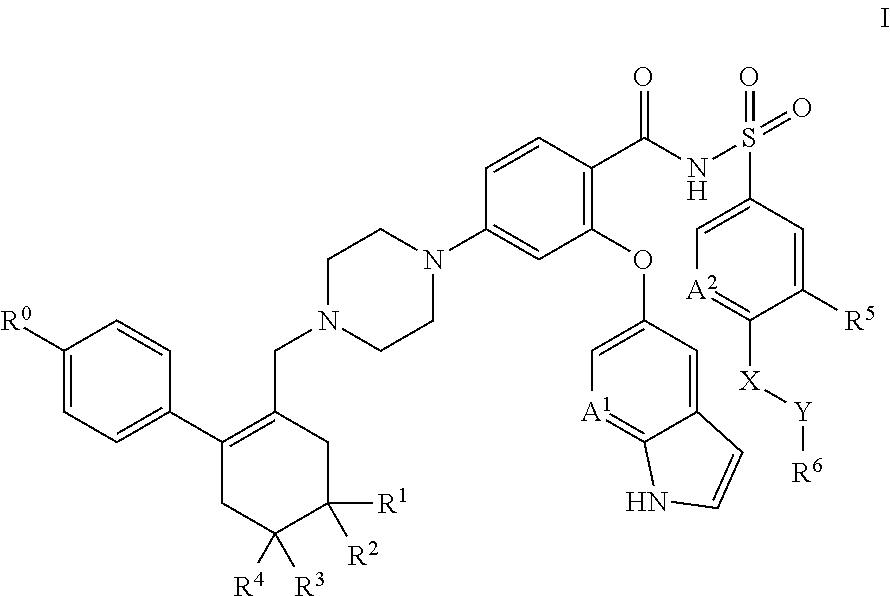
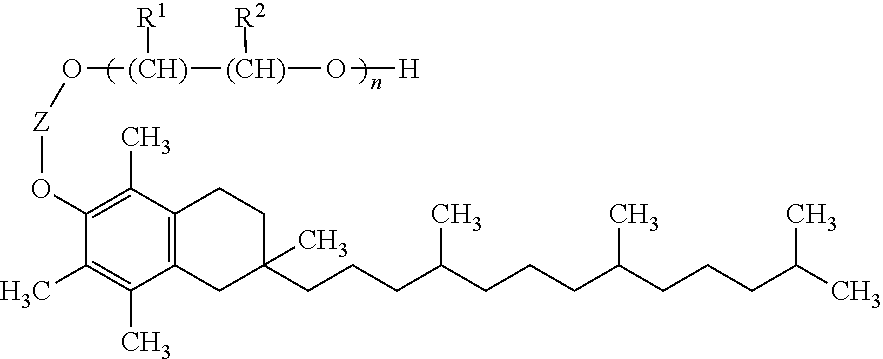
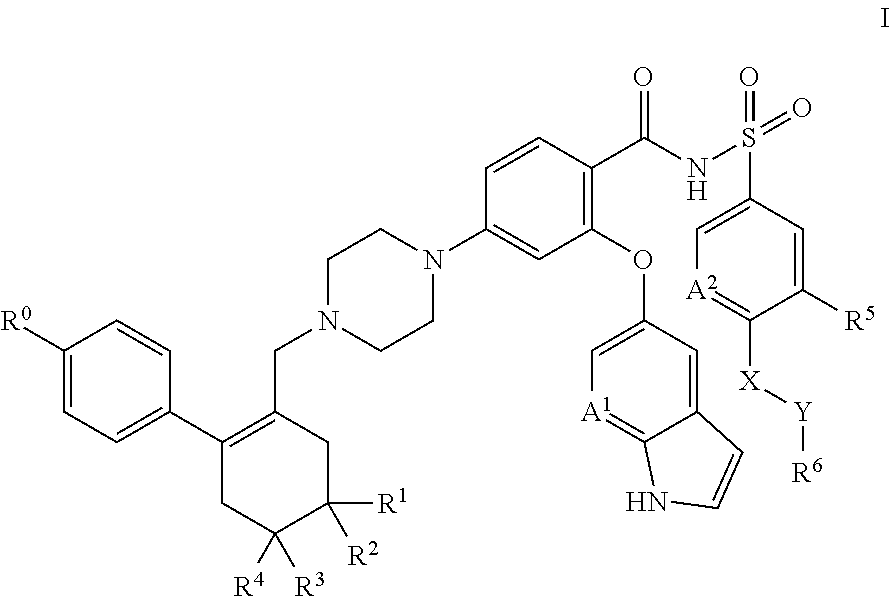
[1369]A solid dispersion comprising API, TPGS surfactant and Soluplus™ polymer in a weight ratio of approximately 21:5:74 is prepared as follows.
[1370]The following solid components are combined in a 100 ml amber glass scintillation vial: 1.0 g API and 3.5 g Soluplus™ polymer. Approximately 45.5 g solvent mixture (THF / water, 90:10) is added to the solids with agitation until the solids are dissolved (this takes approximately 15 minutes), forming a drug / polymer solution. TPGS surfactant (0.05 g) is weighed into a separate 20 ml amber glass scintillation vial, and an aliquot of 10 g of the drug / polymer solution is added to the surfactant, with mixing. The resulting solution is placed in a Genevac rotary evaporator for solvent removal at 80° C. by slowly increasing applied vacuum from ambient to approximately 200 mm Hg over a period of about 3 hours. The powder that remains after solvent removal is collected, with scraping as necessary, from the bottom of the vial, and is screened thro...
example 2
[1371]A solid dispersion comprising API, Tween™ 20 surfactant and Soluplus™ polymer in a weight ratio of approximately 20:10:70 is prepared as in Example 1, except that 0.1 g Tween™ 20 is substituted for 0.05 g TPGS.
example 3
[1372]A solid dispersion comprising API, Tween™ 80 surfactant and Soluplus™ polymer in a weight ratio of approximately 20:10:70 is prepared as in Example 1, except that 0.1 g Tween™ 80 is substituted for 0.05 g TPGS.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com