Genetic medicine for preventing and treating cancer of colon and rectum, preparation process and use thereof
A technology of gene medicine and colorectal cancer, applied in gene therapy, drug combination, pharmaceutical formula, etc., can solve the problems that the treatment methods are difficult to treat effectively, and achieve the effect of reducing toxic side effects and enhancing curative effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
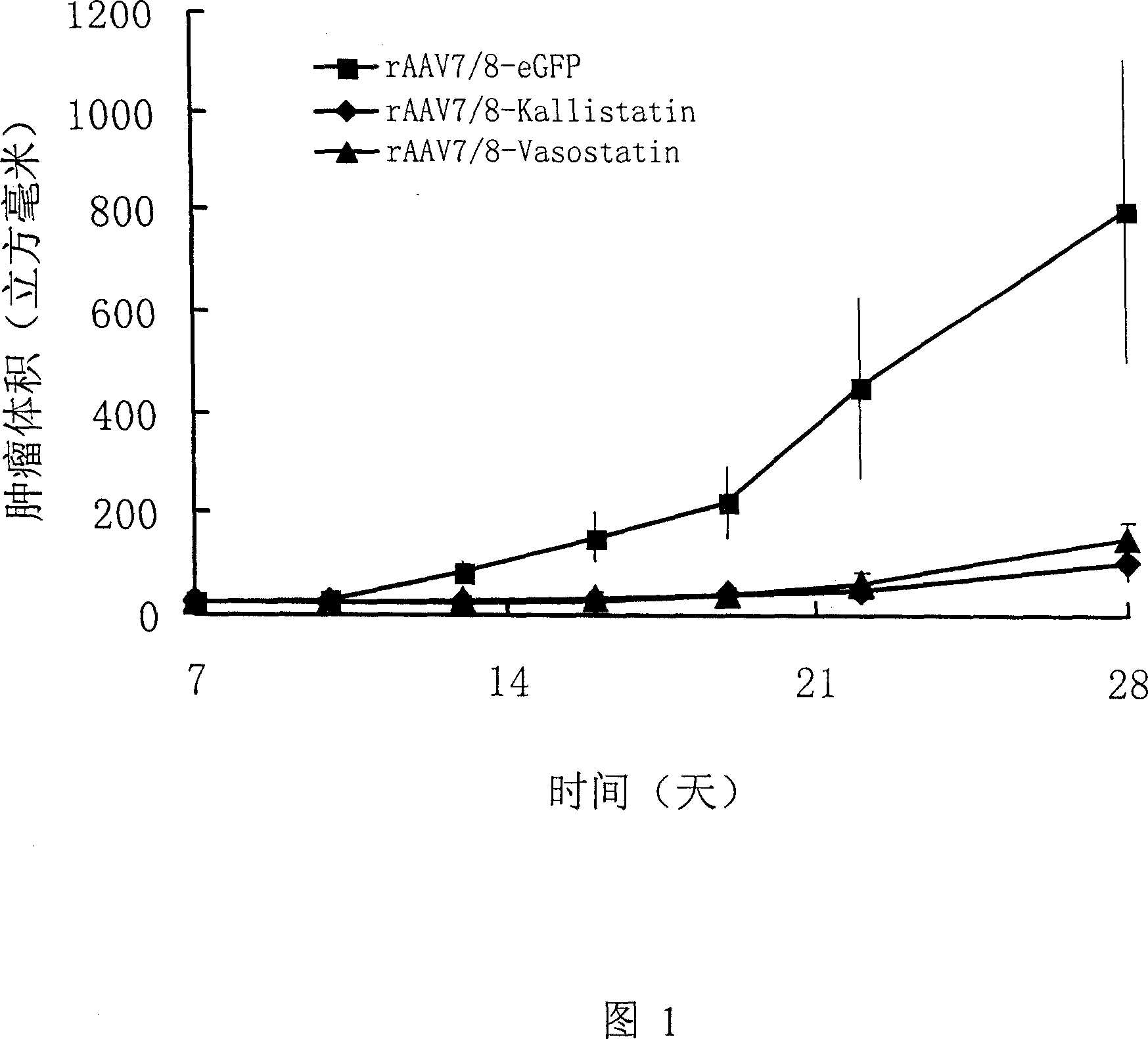
Image
Examples
Embodiment 1
[0046] Preparation of rAAV7 / 8-kallistatin
[0047] Human kallistatin full-length cDNA was amplified from human liver first-strand cDNA by PCR. The exclusive primer for amplifying kallistatin is Kalli-F(5’-AA GAATTC GAGGATGCATCTTATCGAC) and Kalli-R(5’-AA GGTA CCAAGCTT CTATGGTTTCGTGGGGTC). Restriction sites (underlined) were introduced to facilitate subcloning. The PCR conditions were 94°C, 50°C and 68°C for 45 seconds each, with a total of 36 cycles. The PCR product was sequenced to verify its correctness, and subcloned into the AAV-2 expression vector to obtain the rAAV2-kallistatin expression plasmid. The promoter of the expression plasmid is CMV enhancer / chicken β-actin promoter. To enhance expression, after inserting the gene, WPRE elements were also introduced.
[0048] Recombinant virus was prepared by calcium phosphate helper-free virus multi-plasmid method: the expression plasmid, AV helper plasmid, and AAV7 helper plasmid and AAV8 helper plasmid were co-transfected int...
Embodiment 2
[0050] Preparation of rAAV7 / 8-vasostatin
[0051] Human vasostatin full-length cDNA was amplified from human liver first-strand cDNA by PCR. The exclusive primer for amplifying vasostatin is Vas-F(5’-AA CTCGAG CC CGCCATGCTG CTATCC) and Vas-R(5’-AA AAGCTT CTAGTTGTCTGG CCGCACAAT). Restriction sites (underlined) were introduced to facilitate subcloning, and a stop codon (in bold) was introduced in the primer Vas-R. The PCR conditions were 94°C, 50°C and 68°C for 45 seconds each, with a total of 36 cycles. The PCR product was sequenced to verify its correctness, and subcloned into the AAV-2 expression vector to obtain the rAAV2-kallistatin expression plasmid. The promoter of the expression plasmid is CMV enhancer / chicken β-actin promoter. To enhance expression, after inserting the gene, WPRE elements were also introduced.
[0052] Recombinant virus was prepared by calcium phosphate helper-free virus multi-plasmid method: the expression plasmid, AV helper plasmid, and AAV7 helper plas...
Embodiment 3
[0054] Preparation of recombinant virus injection
[0055] Take the recombinant virus rAAV7 / 8-kallistatin 2×10 prepared by the method in Example 1 15The virus titer is obtained by adding normal saline for injection to 1000ml, sterile filtration, and aliquoting into 2ml vials.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com