Acid Salt Forms of Polymer-Drug Conjugates and Alkoxylation Methods
a polymer-drug conjugate and acid salt technology, applied in the field of acid salt compositions, can solve the problems of poor aqueous solubility of pharmaceutical agents, low bioavailability, instability,
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation 1
Preparation of Pentaerythritolyl-4-Arm-(Peg-1-Methylene-2 Oxo-Vinylamino Acetate Linked-Irinotecan)-20K′4-Arm-Peg-Gly-Irino-20K Mixed Acid Salt
[0316]
[0317]All solvents used in synthesis were anhydrous.
Step 1. Conjugation of t-boc-glycine to Irinotecan-HCl salt (>95% yield)
[0318]Irinotecan-HCl-trihydrate (1 mole or 677 g) and DMF (10 L) were charged into a distiller at 60T. Upon dissolution of the irinotecan-HCl-trihydrate in DMF, full vacuum was slowly applied in order to remove water from the irinotecan-HCl-trihydrate by azeotropic distillation at 60° C. Upon solids formation from the residual DMF, heptane (up to 60 L) was charged into the distiller to remove residual DMF at 40-50° C. Upon removal of heptane by visual inspection, the azeotropic distillation was stopped and the solid (irinotecan-HCl) was allowed to cool to 17±2° C. For the coupling reaction, t-hoc-glycine (1.2 mole), 4-DMAP (0.1 mole) dissolved in DCM (1 L), and DCM (19 L) were charged into the distille...
example 2
Characterization of “4-Arm-Peg-Gly-Irino-20K” Product as a Mixed Salt
[0328]The product from Example 1 was analyzed by ion chromatography (IC analysis). See Table 1 below for IC analytical results for various product lots of 4-arm-PEG-Gly-Irino-20K.
TABLE 1Mole Percent of Irinotecan bound to PEGLOT NO.TFA SALTHCl SALTFREE BASE0105936 5 (low)02064 (high)30 603027 (low)244 9 (high)040532621050542620060572815070533314080532720090441936100334126Average of last 7502922lots
[0329]Based upon the IC results provided in Table 1, it can be seen that the product formed in Example 1,4-arm-PEG-Gly-Irino-20K, is a partial mixed salt of approximately 50 mole percent TFA salt, 30 mole percent HCl salt, and 20 mole percent free base, based upon conjugated irinotecan molecules in the product. The mixture of salts was observed even after repeated (1-3) recrystallizations of the product. In the various product lots analyzed above, it can be seen that about 35-65 mole percent of the irinotecan molecules i...
example 3
Stress Stability Studies of 4-Arm-Peg-Gly-Imo-20K
[0331]Stability studies were conducted in an attempt to evaluate the 4-arm-PEG-Gly-Irino-20K product composition. Compositions containing varying amounts of protonated irinotecan, as well as differing in the amount of TFA versus HCl salt were examined.
[0332]Stress Stability Studies
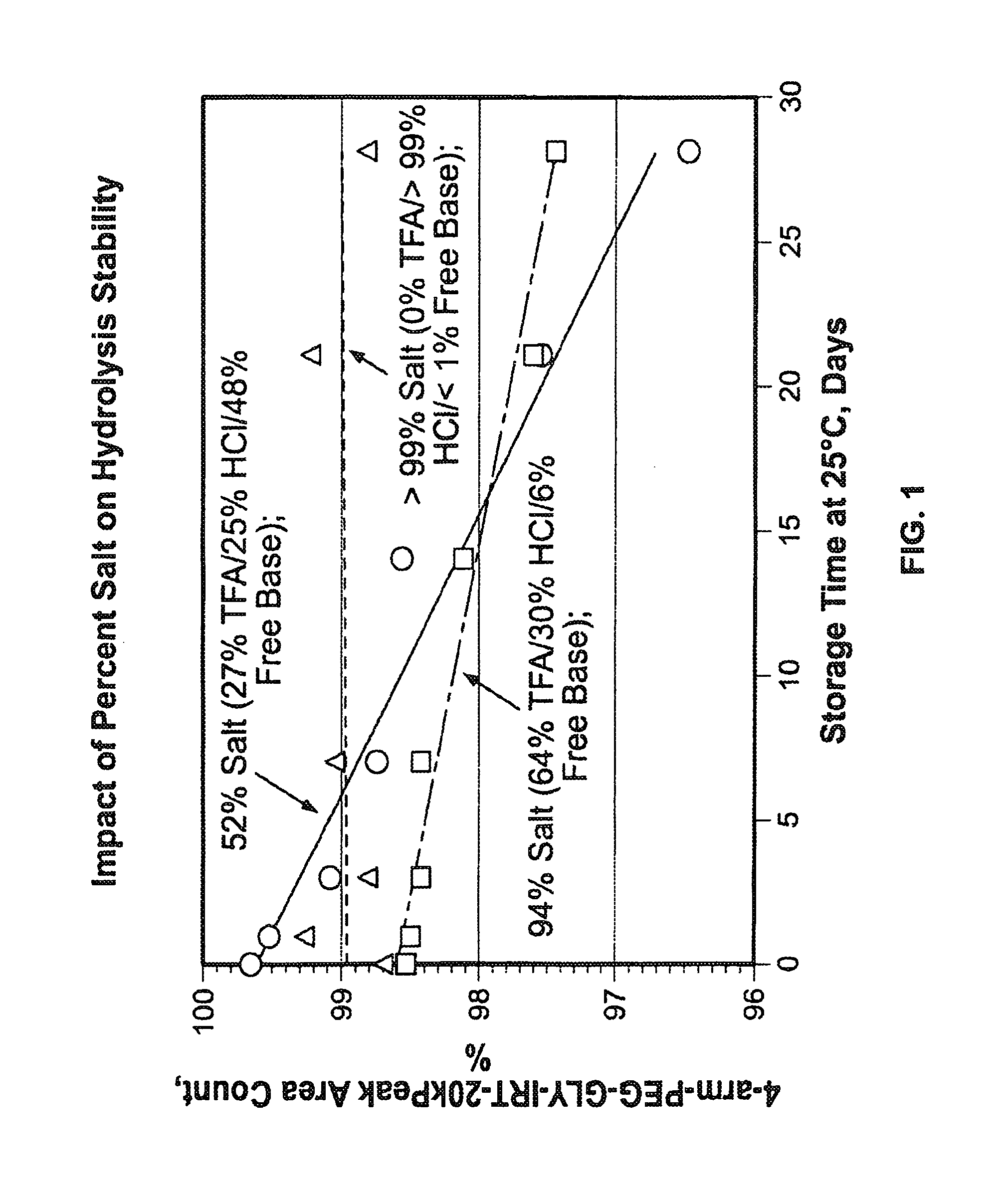
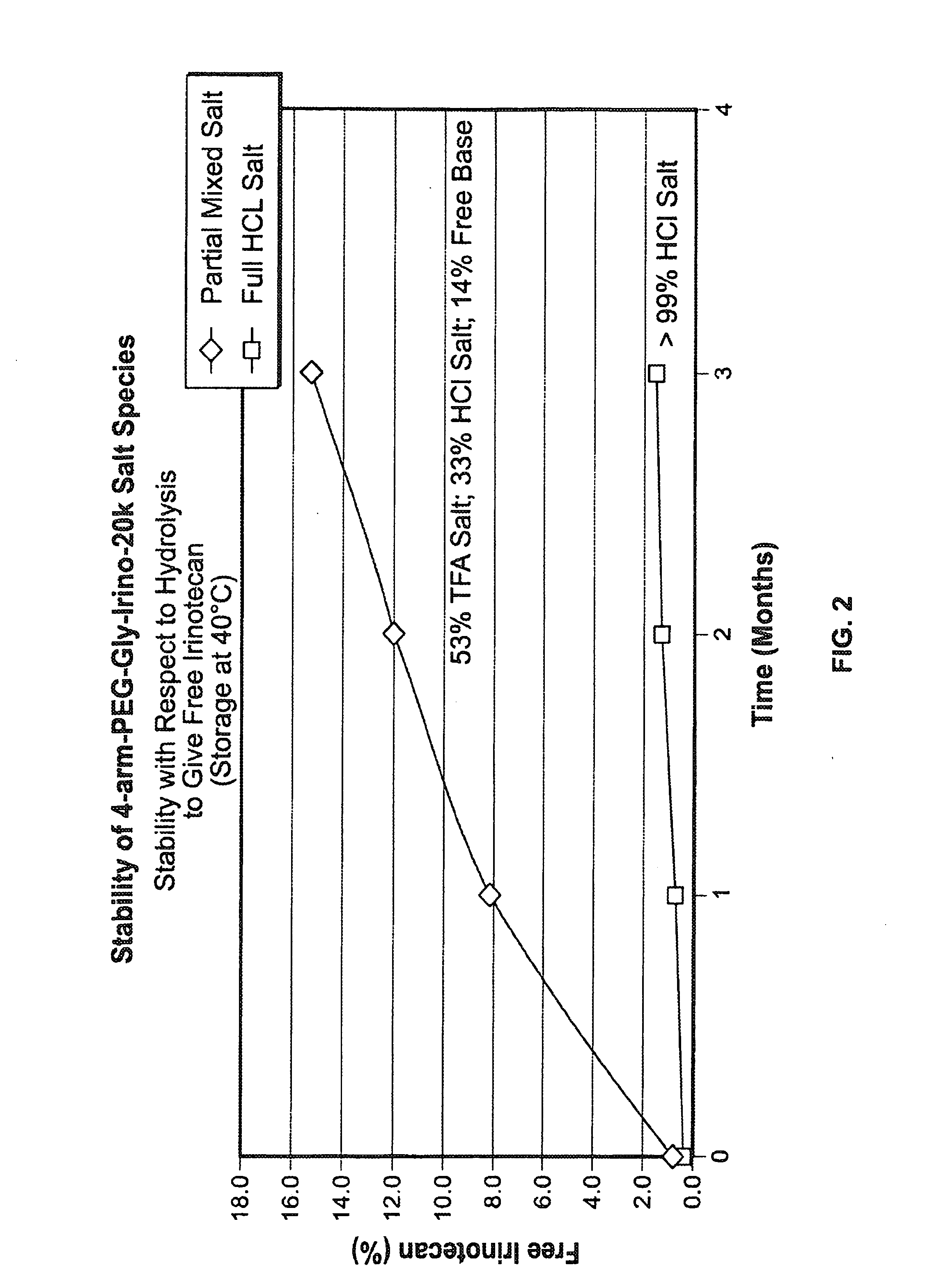
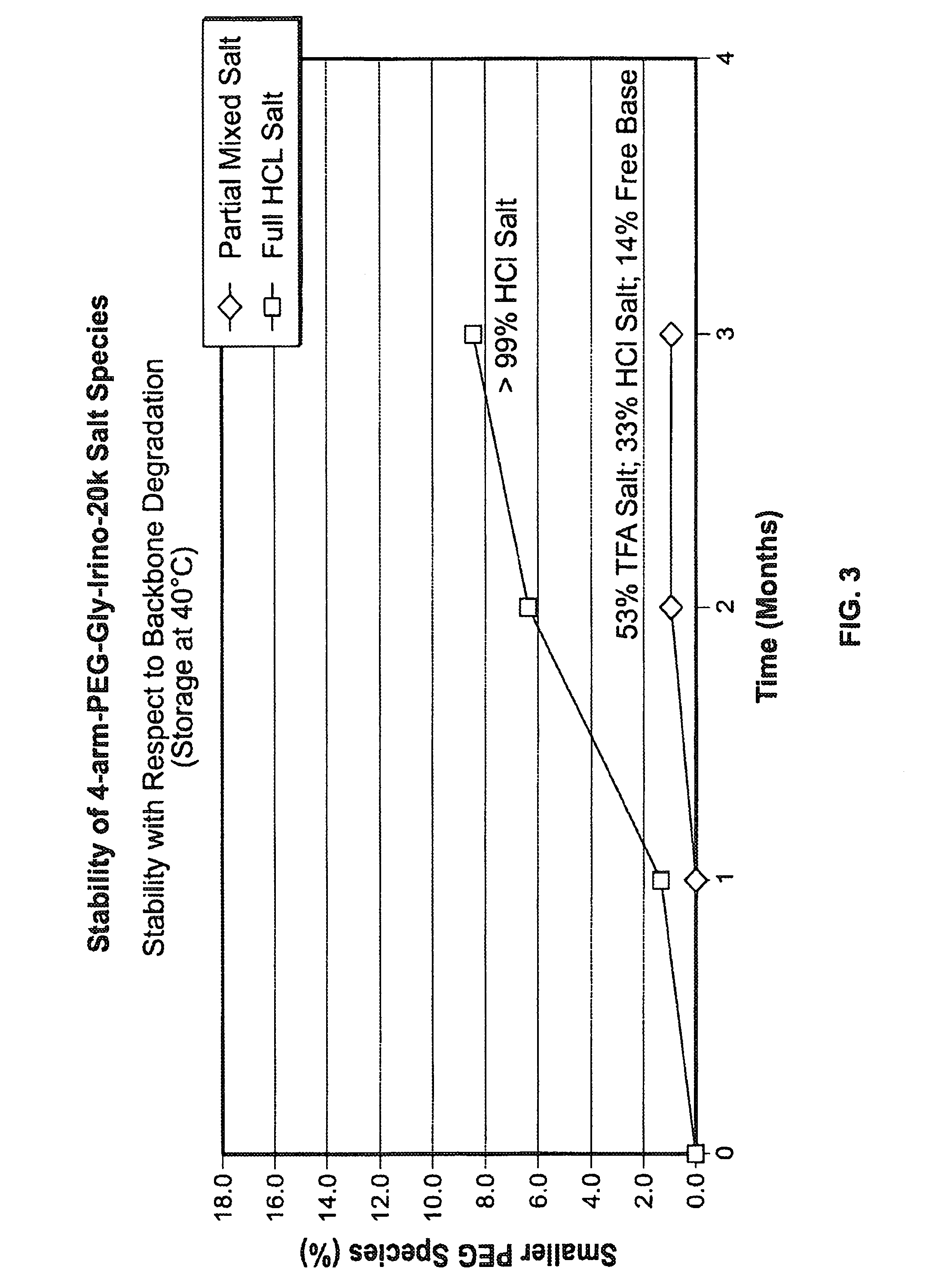
[0333]The product formed in Example 1,4-arm-PEG-Gly-Irino-20K, compound 5. (approximately 1-2 g) was weighed into PEG PE ‘whirl top’ bags and placed into another ‘whirl top’ bag in order to simulate the API packaging conditions. In one study (results shown in FIG. 1), samples were placed in an environmental chamber at 25° C. / 60% RH for 4 weeks. In another study, samples were placed in an environmental chamber at 40° C. / 75% RH for up to several months (results shown in FIG. 2 and FIG. 3). Samples were taken and analyzed on a periodic basis over the course of the studies.
Results
[0334]The results of the studies are shown in FIG. 1, FIG. 2 and FIG. 3. In FIG. 1,...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Mass | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com