Succinic acid solifenacin tablet capable of achieving direct powder compression and preparation method of succinic acid solifenacin tablet
Solifenacin succinate, a direct technology, is applied in the direction of medical preparations with non-active ingredients, medical preparations containing active ingredients, and pill delivery, which can solve problems such as poor fluidity and compressibility, and small doses
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
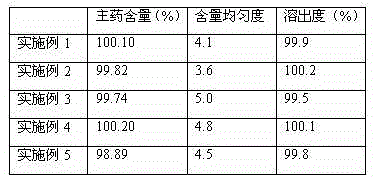
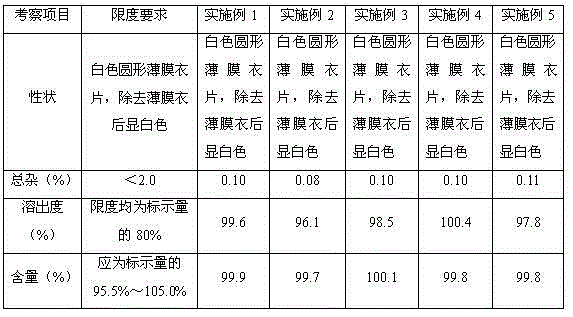
Examples
Embodiment 1-5
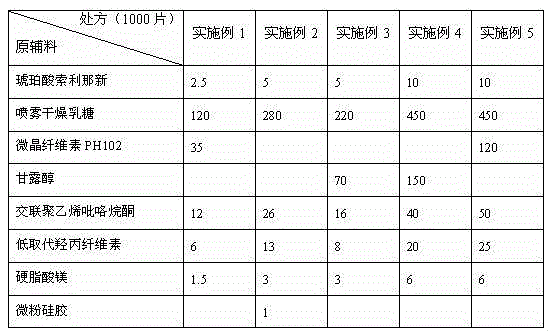
[0022] The prescription quantity of raw and auxiliary materials of solifenacin succinate tablet in embodiment 1-5: (unit: g)
[0023]
[0024] The preparation of the above examples includes the following steps: 1) Take each raw material in proportion, mix the auxiliary materials evenly and pass through an 80-mesh sieve; 2) Mix the main ingredients and auxiliary materials evenly according to the "equal volume increasing method": take all the main ingredients and an equal amount of For excipients, place them in a mixing machine and mix evenly, then add the same amount of excipients as the mixture and mix evenly, so that the amount is increased until the excipients are added, and the mixing time is 10 minutes each time; Direct compression in a high-speed rotary tablet press.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com