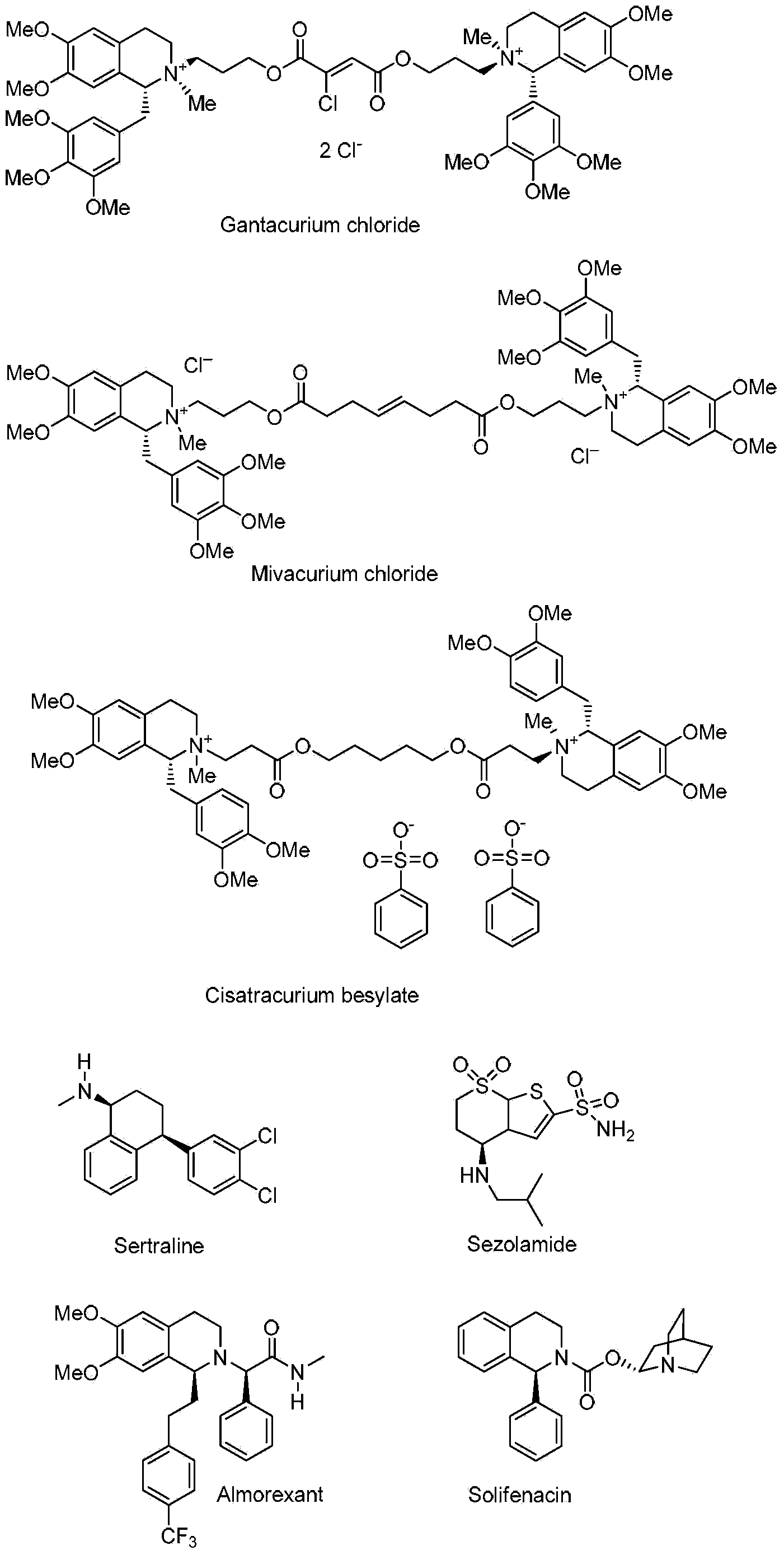
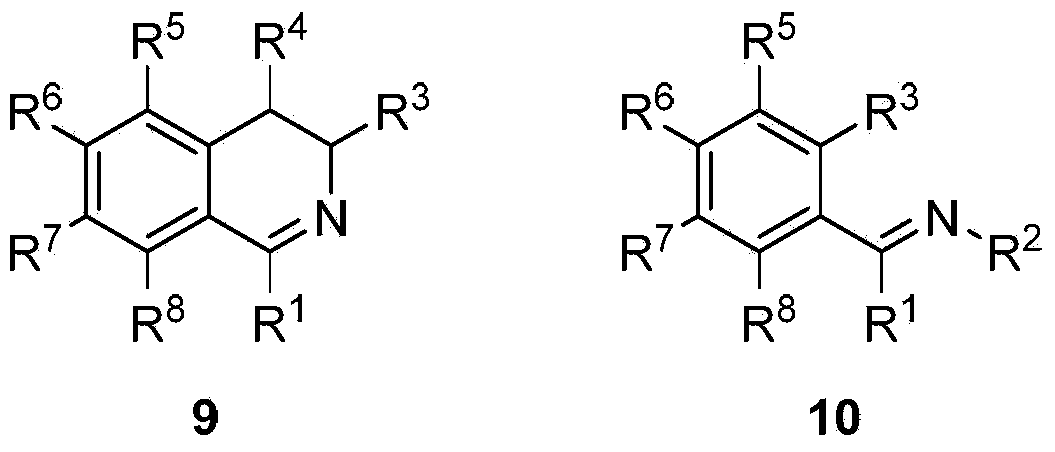
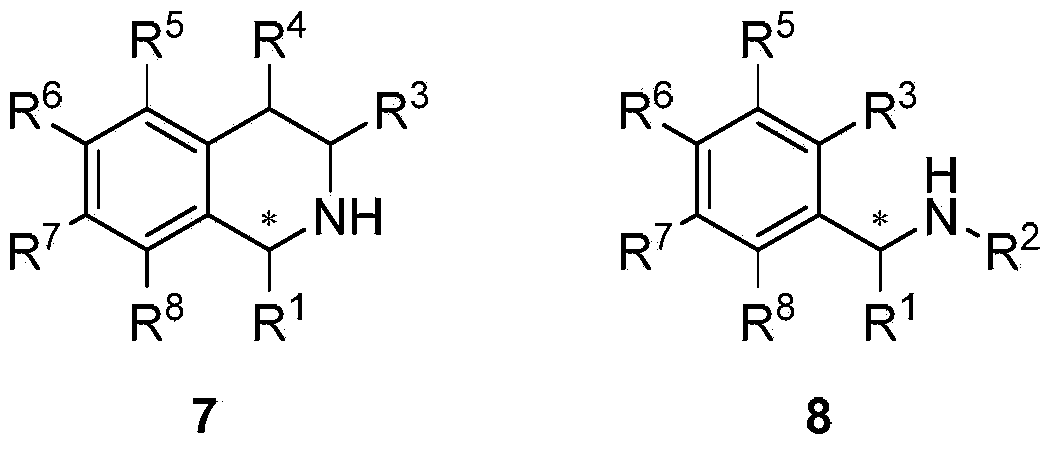
Preparation method and application of chiral amine compound
A technology of imine compounds and compounds, applied in the field of synthesis of pharmaceutical intermediates, can solve problems such as waste
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] The imine 6,7-dimethoxy-1-methyl-3,4-dihydroisoquinoline (1) (1.22mmol) was dissolved in methanol (14.0mL), and trifluoroacetic acid (93.42 μL, 1.22 mmol), and the mixture was stirred for 5 minutes. Catalyst [RuCl(η 6 -p-isopropyltoluene)(S,S)-(N-tosyl-1,2-diphenyl and -1,2-diamine)](A)(0.0122mol) was added to the above mixture ( The molar ratio of substrate to catalyst S / C=100). The reaction system was sealed as a pressure reactor, replaced with hydrogen three times (3×5 bar), and then filled with 15 bar of hydrogen. After reacting for 6 hours, saturated Na 2 CO 3 The solutions were mixed (2 mL). The resulting solution was extracted twice with ether (3×2 mL), and the combined organic phase was washed with anhydrous Na 2 SO 4 Let dry for 1 hour. The resulting ether solution was evaporated to dryness in air vapor. GC analysis showed 1100% conversion of compound 1 to 6,7-dimethyl-1-methyl-1,2,3,4-tetrahydroisoquinoline, ee value 96%.
[0047] Analysis conditions...
Embodiment 2~23
[0050] The operating modes of Examples 2-23 are basically the same as those of Example 1, except that the catalysts and raw materials are different. The catalysts and raw materials used, as well as the conversion ratio and ee value are listed in Table 1.
[0051] The reaction condition and reaction result of table 1 embodiment 2~23
[0052] Example
catalyst
Conversion rates[%]
ee[%]
2
2
A
100
87
3
3
A
100
92
4
4
A
100
93
5
1
B
100
96
6
2
B
100
85
7
3
B
100
92
8
4
B
100
92
9
1
C
100
96
10
2
C
100
81
11
3
C
100
89
12
4
C
100
90
13
1
D
100
78
14
2
D
100
59
15
3
D
100
68
16
4
D
100
74
17
2
E...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com