Pharmaceutical composition containing solifenacin or salt thereof
A technology of solifenacin and solifenacin succinate, which is applied in the field of pharmaceutical compositions containing solifenacin or a salt thereof, can solve the problems of inconvenient mass production, high requirements for testing equipment, etc., and achieves improved production efficiency, Simple operation process and improved stability effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
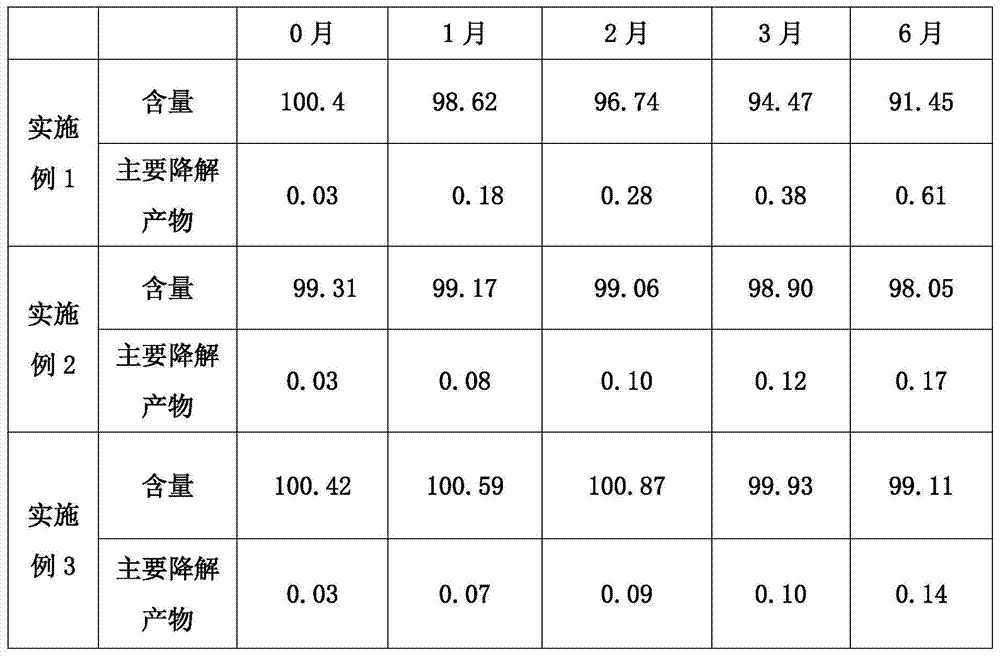
Embodiment 1
[0019] Embodiment 1: a kind of pharmaceutical composition containing solifenacin or its salt, it comprises following composition by weight ratio:
[0020] Element
weight%
Features
Solifenacin Succinate
3.33
78.77
filler
13.90
filler
3.00
1.00
[0021] The percentages in the above formulations are calculated for tablets containing 5 mg of solifenacin succinate.
[0022] Weigh hypromellose according to the prescription amount, add water to prepare a solution with a concentration of 15% (w / v), and use it as binder solution A.
[0023] Weigh solifenacin succinate, lactose and cornstarch according to the prescription amount, mix them uniformly according to the method of equal volume addition, granulate with the above solution A, ventilate and dry at 55±5°C, and add stearic acid after the dry ...
Embodiment 2
[0024] Embodiment 2: a kind of pharmaceutical composition containing solifenacin or its salt, it comprises following composition by weight ratio:
[0025] Element
weight%
Features
3.33
0.10
78.67
filler
13.90
filler
3.00
1.00
[0026] The percentages in the above formulations are calculated for tablets containing 5 mg of solifenacin succinate.
[0027] Weigh hypromellose according to the prescription amount, add water to prepare a solution with a concentration of 15% (w / v), and use it as binder solution A.
[0028] Weigh solifenacin succinate, lactose, butylated hydroxyanisole and cornstarch according to the prescription amount, mix them uniformly according to the method of equal volume addition, granulate with the...
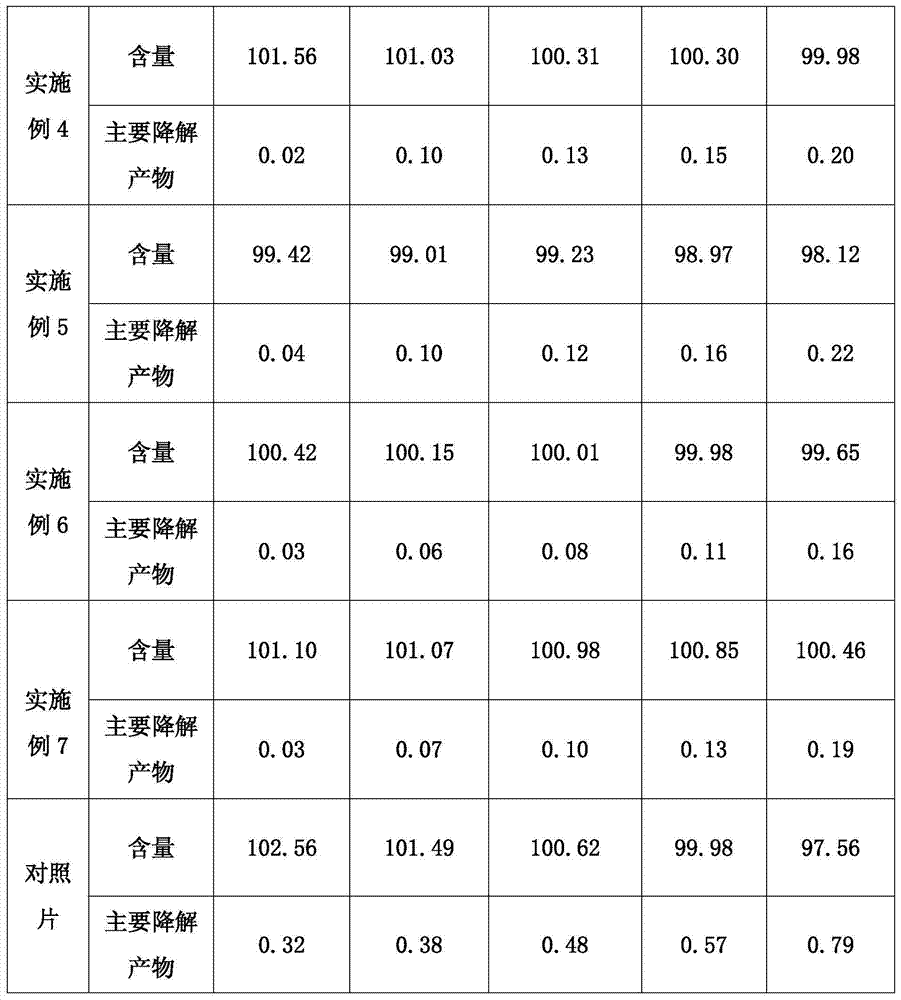
Embodiment 3
[0029] Embodiment 3: a kind of pharmaceutical composition containing solifenacin or its salt, it comprises following composition by weight ratio:
[0030] Element
weight%
Features
3.33
2,6 di-tert-butyl-p-cresol
0.10
antioxidant
lactose
78.67
filler
13.90
filler
hypromellose
3.00
1.00
[0032] The percentages in the above formulations are calculated for tablets containing 5 mg of solifenacin succinate.
[0033] Weigh hypromellose according to the prescription amount, add water to prepare a solution with a concentration of 15% (w / v), and use it as binder solution A.
[0034] Weigh solifenacin succinate, lactose, 2,6-di-tert-butyl-p-cresol and cornstarch according to the prescription amount, mix them uniformly according to the method of equal volume addit...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com