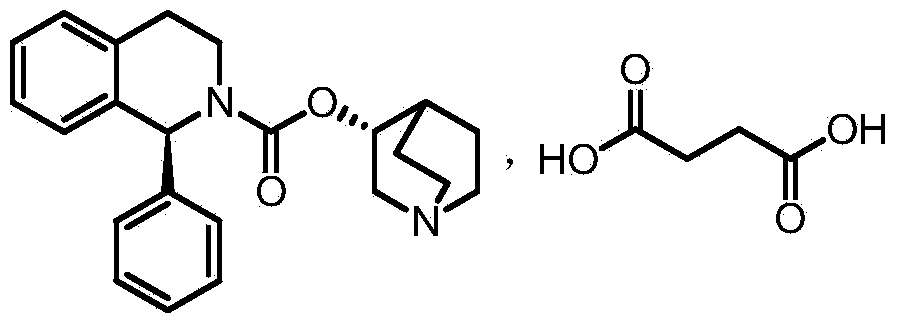
(1S)-1-phenyl-3,4-dihydro-1H-isoquinoline-2-phenyl formate novel crystal form and preparation method thereof
A technology of phenyl formate and tetrahydroisoquinoline, which is applied in the field of medicine and chemical industry, and achieves the effects of mild conditions, easy reaction and low toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
[0051] Preparation Example 1: Preparation of crude (1S)-1-phenyl-3,4-dihydro-1H-isoquinoline-2-carboxylic acid phenyl ester:
[0052] This preparation example is obtained with reference to the document US20090203915A1;
[0053] Dissolve 35g (167mmol) of (S)-1-phenyl-1,2,3,4-tetrahydro-isoquinoline in 175ml of toluene, add 35.8g (167mmol) of diphenyl carbonate and a catalytic amount of dimethyl Aminopyridine was then refluxed. After the reaction was complete, the reactant was cooled, and the resulting reaction solution was washed with 5% NaOH, 1M hydrochloric acid and saturated brine, respectively, and the solvent was distilled off under reduced pressure to obtain compound I as a yellow oil.
Embodiment 1
[0054] Example 1: Preparation of (1S)-1-phenyl-3,4-dihydro-1H-isoquinoline-2-carboxylic acid phenyl ester crystal form A
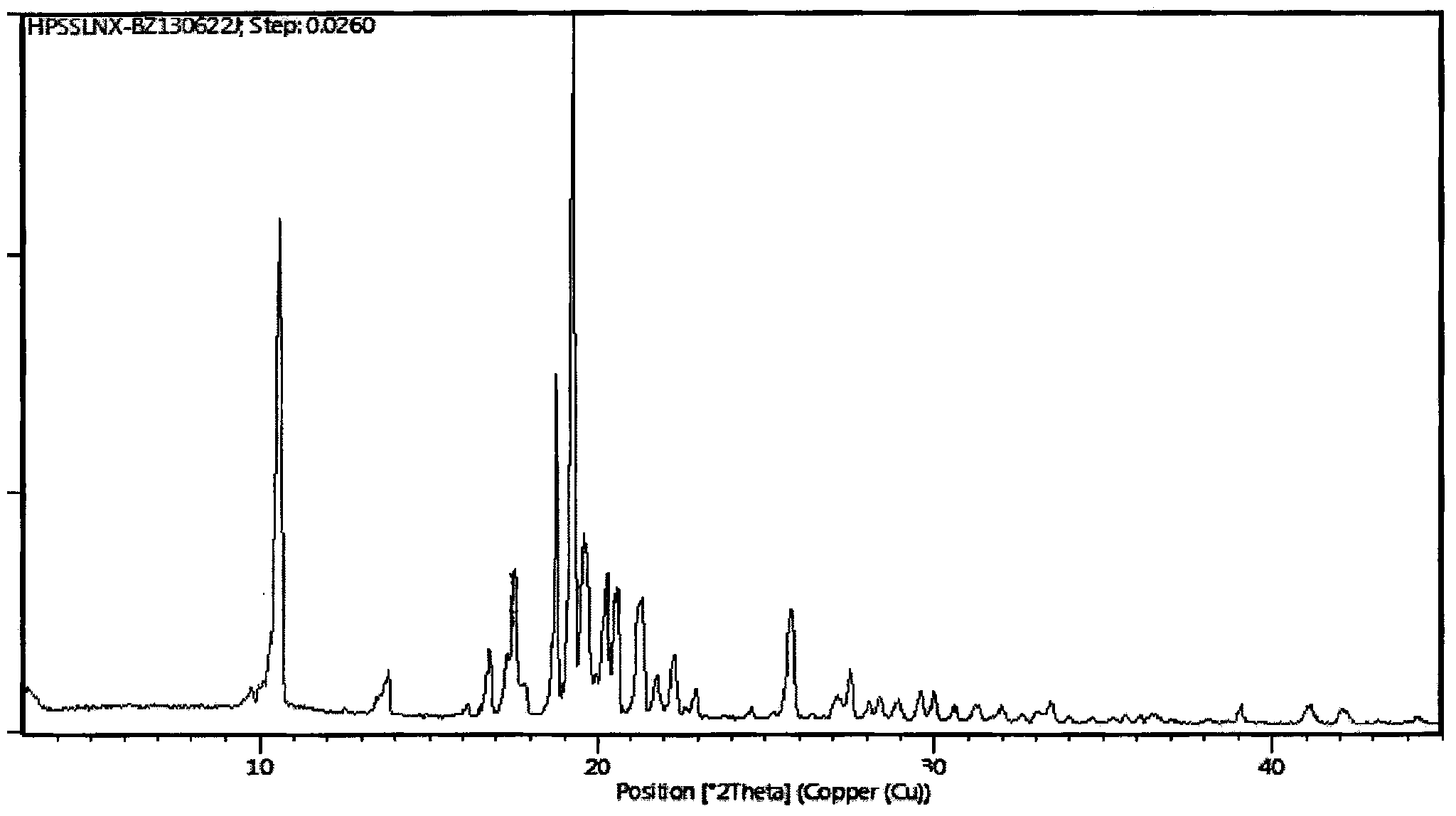
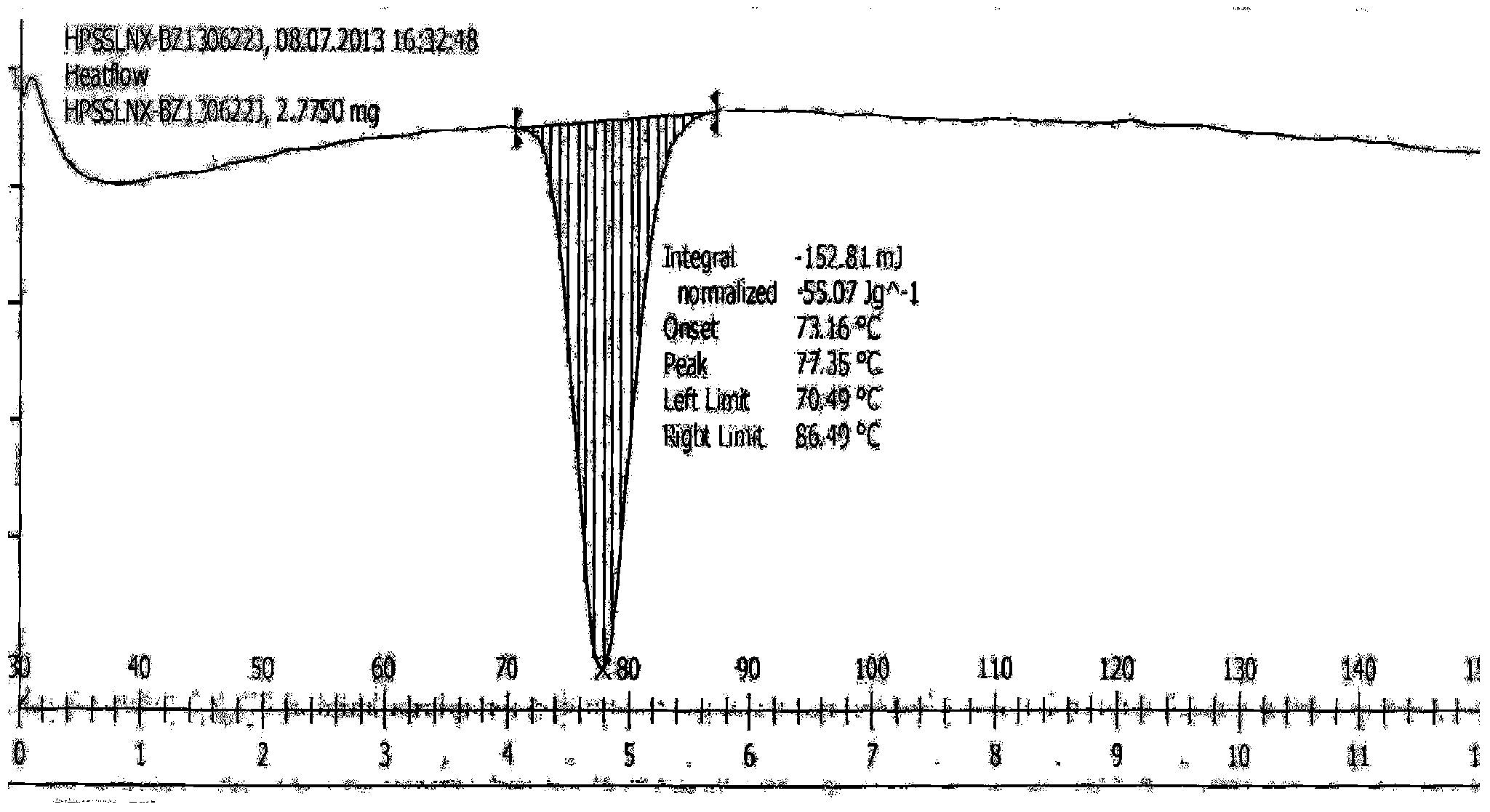
[0055] Add 10 g of the crude compound I obtained in Preparation Example 1 into 50 ml of n-heptane, stir and heat to reflux to dissolve, cool down to crystallize, filter with suction, and dry to obtain 9.8 g of a white solid with a yield of 98% and a purity of 100%. After measurement, the X-ray powder diffraction pattern of gained product is as follows figure 1 As shown, its differential scanning analysis (DSC) pattern is as follows figure 2 shown.
Embodiment 2
[0056] Example 2: Preparation of (1S)-1-phenyl-3,4-dihydro-1H-isoquinoline-2-carboxylic acid phenyl ester crystal form A
[0057] Add 10 g of the crude compound I obtained in Preparation Example 1 into 20 ml of petroleum ether, stir and heat to reflux to dissolve, cool down to crystallize, filter with suction, and dry to obtain 9.7 g of a white solid with a yield of 97% and a purity of 99.9%; The X-ray powder diffraction pattern of gained product and figure 1 Basically the same, its DSC spectrum and figure 2 Basically the same.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com