Stable particular pharmaceutical composition of solifenacin or salt thereof
A technology of solifenacin and composition, applied in the field of stable granular pharmaceutical composition of solifenacin or its salt
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
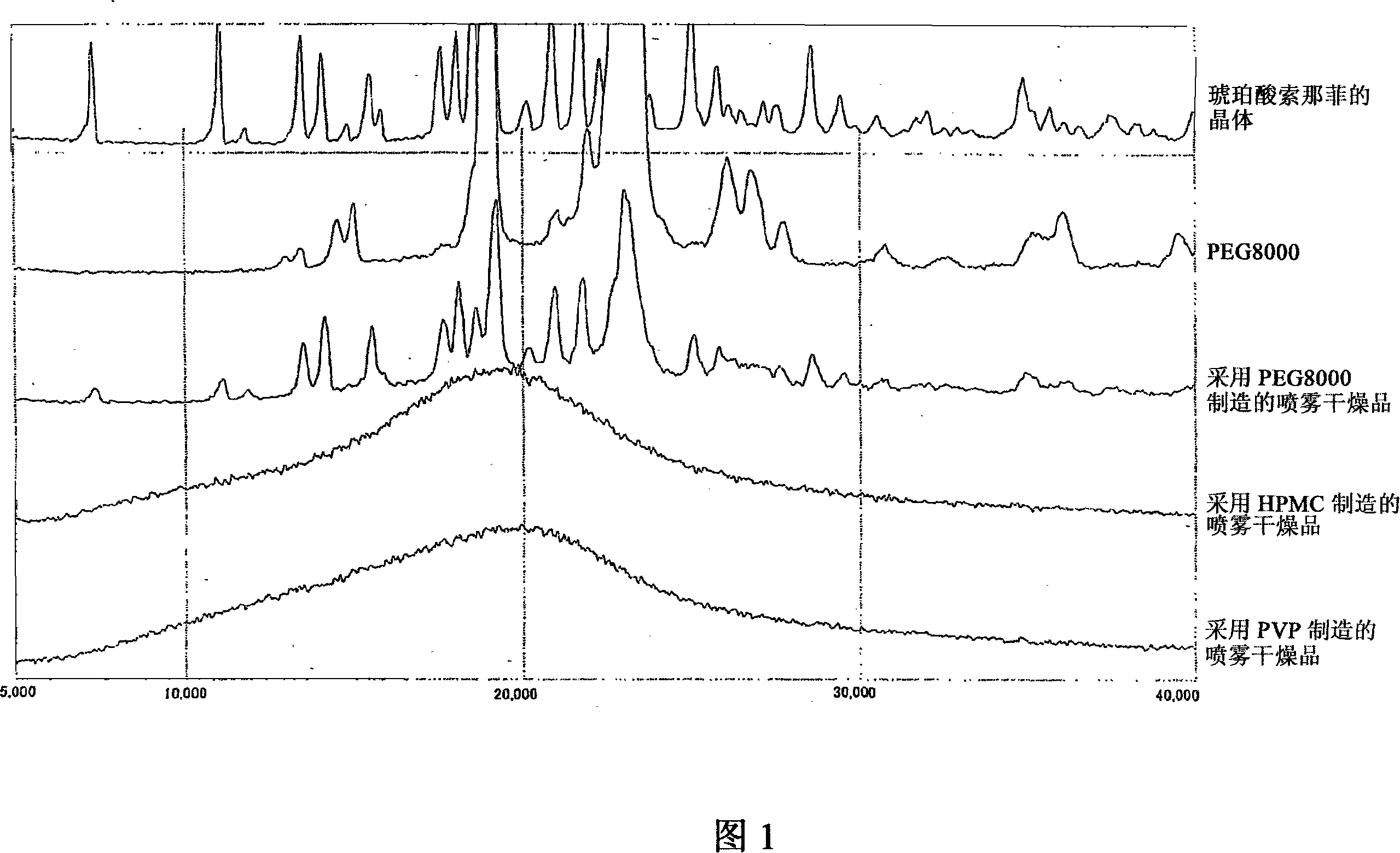
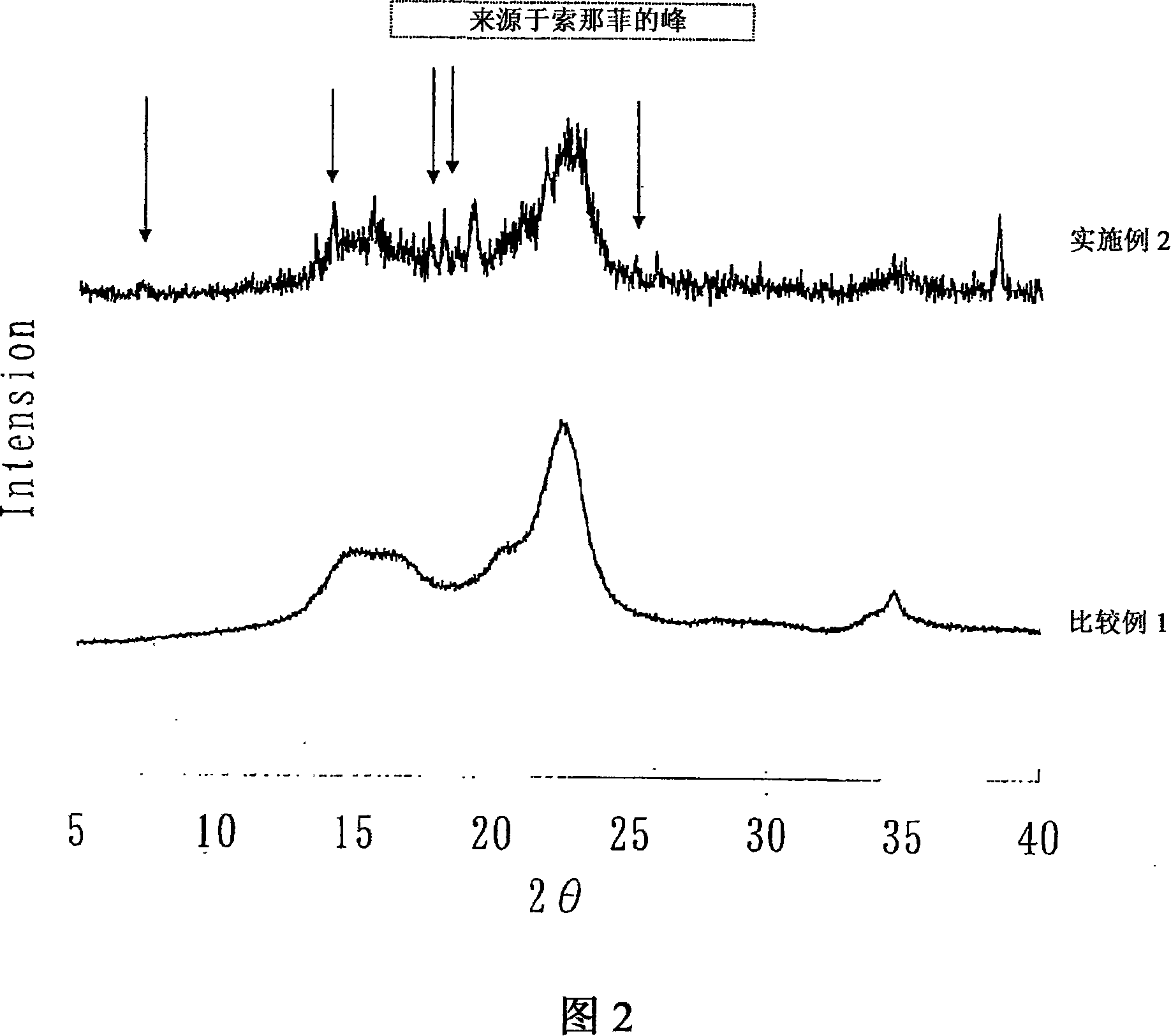
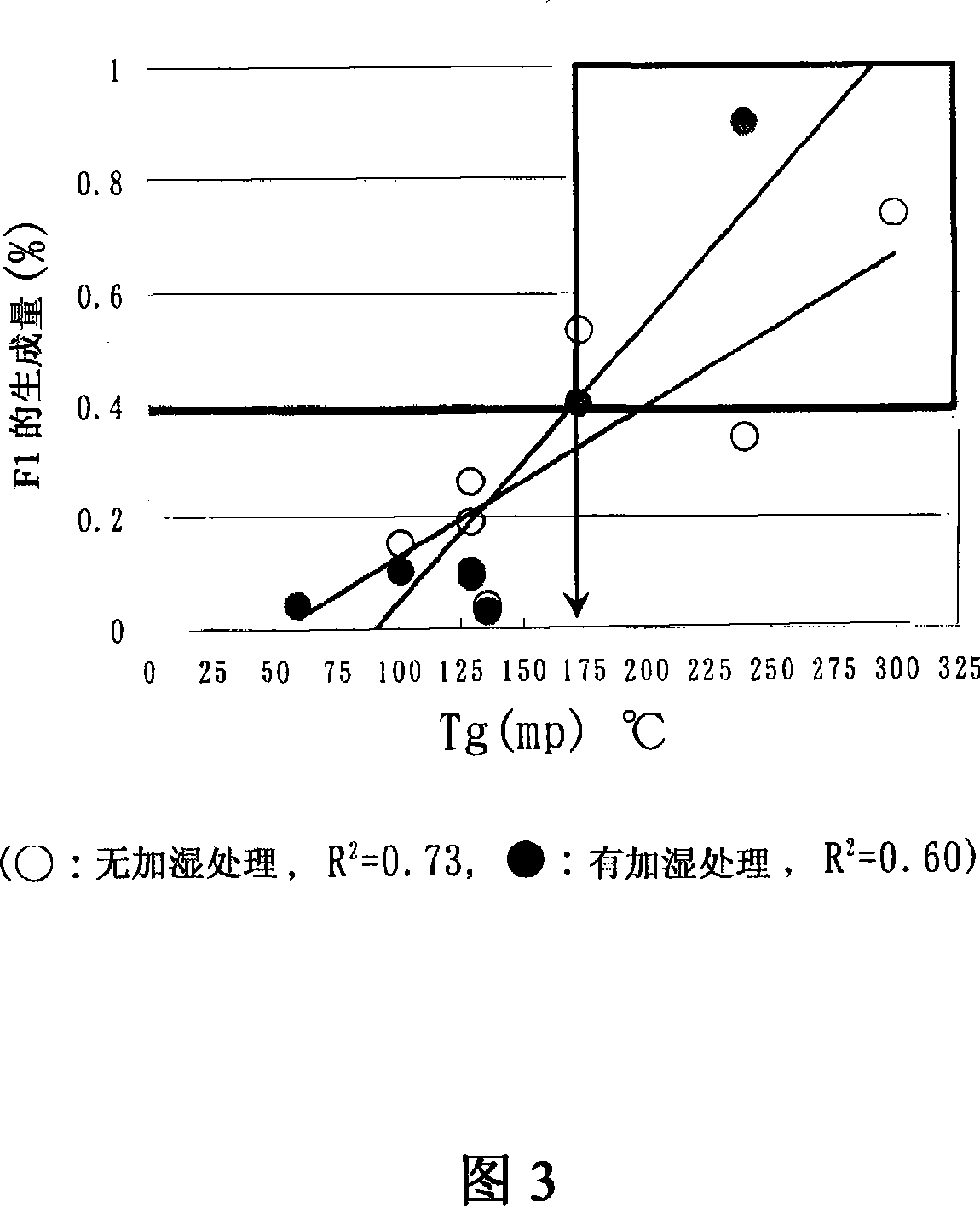
Image
Examples
Embodiment 1
[0091] A substance obtained by coating solifenacin succinate on crystalline cellulose core particles using HPC-SL as a binder
[0092] 10 parts of solifenacin succinate, 3.4 parts of hydroxypropyl cellulose (trade name HPC-SL, manufactured by Nippon Soda Co., Ltd., hereinafter referred to as HPC) are added in the mixed solution of 26.6 parts of water and 26.6 parts of methanol, and stirred (MGM-66, manufactured by SHIBATA) was stirred and dissolved to prepare a drug solution. Next, 60 parts of crystalline cellulose (trade name Celphere, manufactured by Asahi Kasei) was added to a fluidized bed granulator (FLO-1, manufactured by Gratto Co., Ltd.). 3 / min, adhesive liquid spray speed 4.0g / min, spray air pressure 3.0kg / cm 2 Under the conditions, the aforementioned drug solution is sprayed on the Celphere to obtain the granular composition of the present invention.
Embodiment 2
[0094] The granular composition obtained in Example 1 was humidified at 25° C. and 75% RH for 12 hours, further dried at 30° C. and 40% RH for 3 hours, and crystallized to obtain the granular composition of the present invention.
Embodiment 3
[0096] Using PEG6000 as a binder, the material obtained by coating solifenacin succinate on crystalline cellulose core particles
[0097] Add 10 parts of solifenacin succinate and 3.4 parts of polyethylene glycol (trade name Macrogol 6000, manufactured by Sanyo Chemical Industry) to a mixed solution of 26.6 parts of water and 26.6 parts of methanol, and use a stirrer (MGM-66, manufactured by SHIBATA) Stir to dissolve and prepare a drug solution. Next, 60 parts of Celphere (manufactured by Asahi Kasei) was added to a fluidized bed granulator (FLO-1, manufactured by Gratto Co., Ltd.) 3 / min, adhesive liquid spray speed 10g / min, spray air pressure 3.0kg / cm 2 Under the conditions, the aforementioned drug solution is sprayed on the Celphere to obtain the granular composition of the present invention.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com