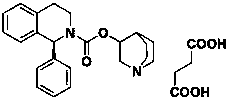
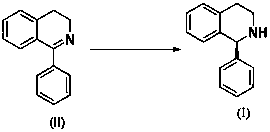
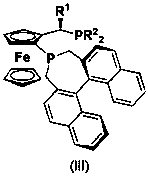
Asymmetric synthesis method of Solifenacin intermediate
A technology for solifenacin and intermediates, which is applied in the field of preparation of solifenacin intermediates, can solve the problems of cumbersome splitting steps, unsatisfactory enantioselectivity control and the like, and achieves a simple preparation method and high efficiency Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027]
[0028] Under nitrogen protection, [Ir(COD)Cl] 2 (1.6 mg, 0.0024 mmol) and the above bisphosphine ligand (3.6 mg, 0.0055 mmol) and 1 mL of tetrahydrofuran were placed in a Schlenk reaction tube, stirred for 30 min, the catalyst solution was transferred to the hydrogenation reaction tube, and 104 mg Imine substrate and 7 μL hydrobromic acid aqueous solution (40%), then add 1 mL tetrahydrofuran, hydrogen replacement three times, at room temperature and 50 atm H 2 After the reaction was completed, the solvent was evaporated to dryness, and the residue was dissolved in 10 mL of dichloromethane, washed with saturated sodium bicarbonate solution, water and brine successively, dried over anhydrous sodium sulfate, and the solvent was evaporated to dryness, and then a short silica gel column Column chromatography obtains solifenacin intermediate I, 1 H NMR detection reaction conversion > 99 %, chiral HPLC detection enantiomeric excess > 99 % ee . 1 H NMR (400 MHz, CDCl 3...
Embodiment 2
[0030]
[0031] Under nitrogen protection, [Ir(COD)Cl] 2 (1.6 mg, 0.0024 mmol) and the above bisphosphine ligand (4.2 mg, 0.0055 mmol) and 1 mL of tetrahydrofuran were placed in a Schlenk reaction tube, stirred for 30 min, the catalyst solution was transferred to the hydrogenation reaction tube, and 104 mg Imine substrate and 7 μL hydrobromic acid aqueous solution (40%), then add 1 mL tetrahydrofuran, hydrogen replacement three times, at room temperature and 50 atm H 2 After the reaction was completed, the solvent was evaporated to dryness, and the residue was dissolved in 10 mL of dichloromethane, washed with saturated sodium bicarbonate solution, water and brine successively, dried over anhydrous sodium sulfate, and the solvent was evaporated to dryness, and then a short silica gel column Column chromatography obtains solifenacin intermediate I, 1 H NMR detection reaction conversion rate > 99 %, chiral HPLC detection enantiomeric excess > 84 % ee .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com