Two crystal forms of sertraline citrate, and preparation methods thereof
A technology for sertraline citrate and crystal form, which is applied in the research field of preparing sertraline citrate crystal form and its synthesis process, can solve problems such as not being approved for listing, and achieves the advantages of being beneficial to storage, less steps, and easier to operate. easy effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Embodiment 1: Preparation of Sertraline Citrate Form A:
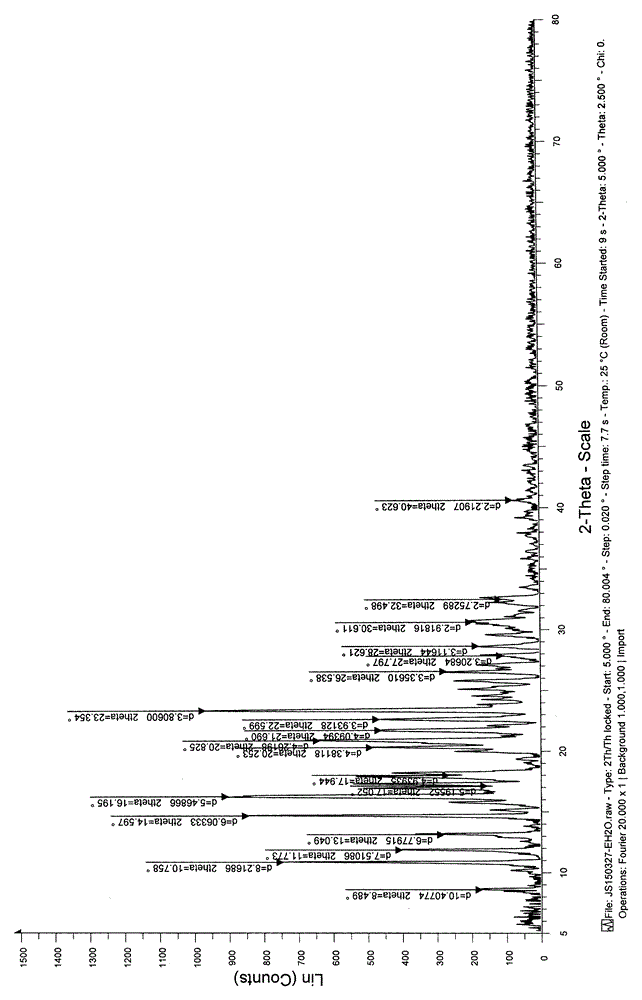
[0032] Take 50.0g of sertraline citrate, add 200ml of 95% ethanol, heat to 60-70°C to dissolve until clear, keep for 10min, stop heating and add 6000ml of water dropwise, cool down to room temperature, keep stirring for 24h, filter, and decompose at room temperature. The sertraline citrate crystalline powder obtained was dried under pressure for 12 hours. The X-ray powder diffraction measurement shows that the obtained crystal form is crystal form A. The specific peak positions are shown in Table 1 below.
[0033] Table 1: X-ray powder diffraction data of sertraline citrate crystal form A in Example 1 of the present invention
[0034]
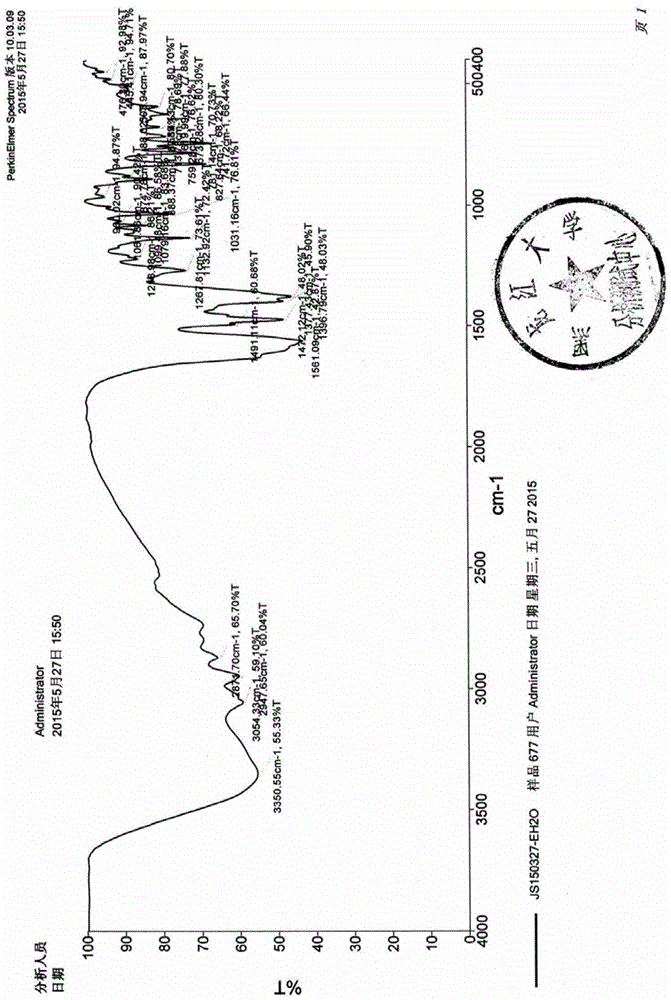
[0035] Other tests were carried out on the obtained samples, and the obtained infrared spectra were as figure 2 shown.
[0036] The obtained samples were tested for solubility, and the results were: Sertraline citrate crystal form A sample was easily soluble in ethanol (...
Embodiment 2
[0037] Embodiment 2: Preparation of sertraline citrate crystal form B:
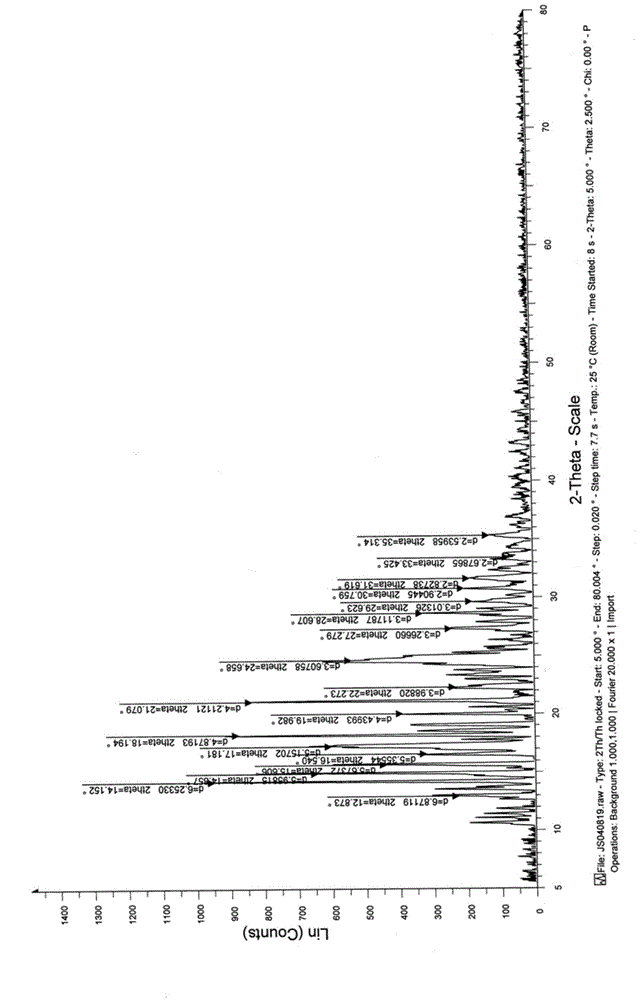
[0038]Take 50.0g of sertraline citrate, add 500ml of 95% ethanol, heat to 60-70°C to dissolve until clear, keep for 10min, stop heating and cool down to room temperature, keep stirring for 24h, filter, dry under reduced pressure at room temperature for 12 hours to obtain Sertraline citrate crystalline powder. The X-ray powder diffraction measurement shows that the obtained crystal form is crystal form B. The specific peak positions are shown in Table 2 below.
[0039] Table 2: X-ray powder diffraction data of sertraline citrate crystal form B in Example 2 of the present invention
[0040]
[0041] Other tests were carried out on the obtained samples, and the obtained infrared spectra were as Figure 4 shown.
[0042] The obtained samples were tested for solubility, and the results were: Sertraline citrate crystal form B samples were dissolved in ethanol (1%, g / ml), very slightly dissolved in 0...
Embodiment 3
[0043] Embodiment 3: Preparation of sertraline citrate crystal form A:
[0044] Take 50.0g of sertraline citrate, add 500ml of 95% ethanol, heat to 60-70°C to dissolve until clear, keep for 10min, stop heating and add 10000ml of water dropwise, lower to room temperature, keep stirring for 24h, filter, and decompose at room temperature The sertraline citrate crystalline powder obtained was dried under pressure for 12 hours. The X-ray powder diffraction measurement shows that the obtained crystal form is consistent with the crystal form of Example 1. The specific peak positions are shown in Table 1 below.
[0045] Table 3: X-ray powder diffraction data of sertraline citrate crystal form A in Example 3 of the present invention
[0046]
[0047] The obtained sample is carried out infrared test, and the obtained infrared spectrum is consistent with figure 2 unanimous.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com