Sertraline side chain amino structure derivative as well as preparation method and application thereof
A technology of chain amino and sertraline, applied in the field of medicine, can solve problems such as chemotherapeutic drug resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Embodiment 1: the synthesis of compound
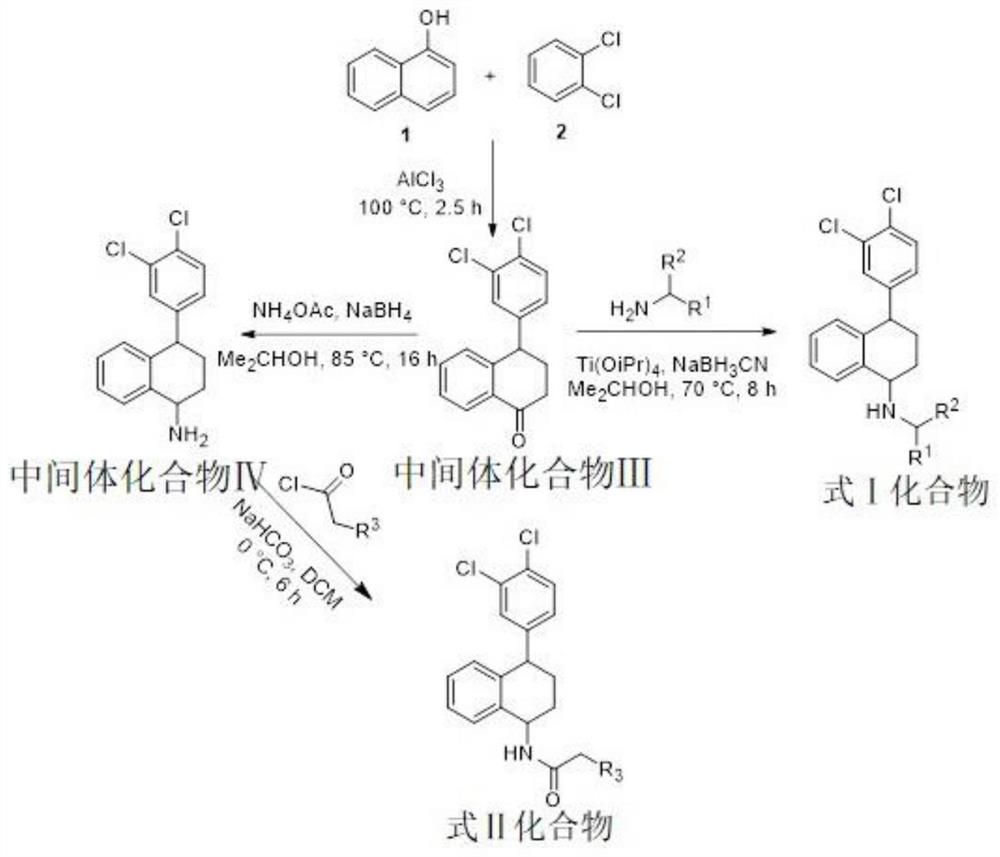
[0049] According to the synthesis process of sertraline side chain amino structure derivatives of the present invention figure 1 As shown, weigh compound 1 and aluminum trichloride in a three-necked flask, under N 2Add compound 2 to it under protected conditions, and raise the temperature to 100°C to react for 2.5 hours. After the reaction is complete, cool to room temperature and quench with a mixture of 1N hydrochloric acid and ice, then extract with dichloromethane, and then carry out conventional water removal operations , after being spin-dried, carry out column chromatography separation to obtain the intermediate compound; the equivalent ratio of compound 1 to aluminum trichloride and compound 2 is: 1:8.157 ~ 1:1:2.5.
[0050] 1, the synthetic method of the sertraline derivative shown in formula I
[0051]
[0052] The intermediate compound III was dissolved in isopropanol solution, N 2 Add the modifying group compo...
Embodiment 2
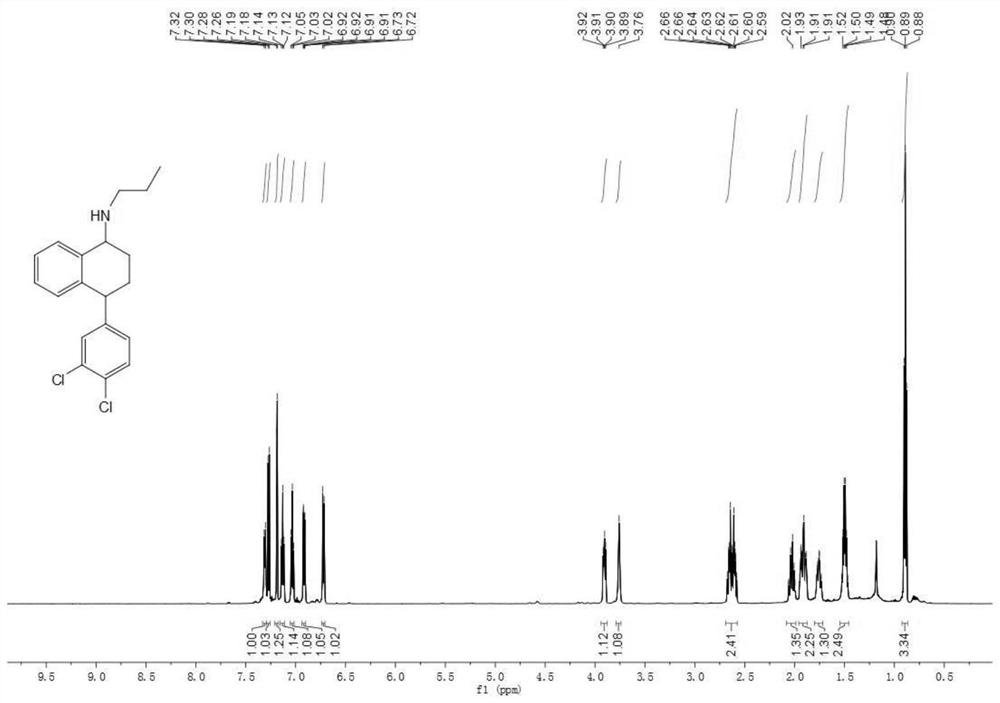
[0065] The synthesis of embodiment 2 numbering NO.1 sertraline derivatives
[0066] The weighed intermediate compound III (583.9mg (2mmol)) was placed in a dry three-necked round-bottomed flask with a stirring bar, and anhydrous and anaerobic treatment was carried out, and then n-propylamine (0.33ml (4mmol)) was added thereto and stirred for 5 Minutes later, tetraisopropyl titanate (2ml (6.75mmol)) was added thereto and refluxed in an oil bath at 85 degrees Celsius for 2 hours. After 2 hours, it was cooled to room temperature and then sodium cyanoborohydride was added thereto. (314.2mg (5mmol)) and heated to 85°C and refluxed until the reaction was complete. After the reaction is complete, the reaction solution is cooled to room temperature, and then quenched by adding an appropriate amount of saturated sodium bicarbonate solution, then extracted with dichloromethane, dried with anhydrous sodium sulfate, suction filtered, and spin-dried to obtain Yellow oil; followed by colum...
Embodiment 3
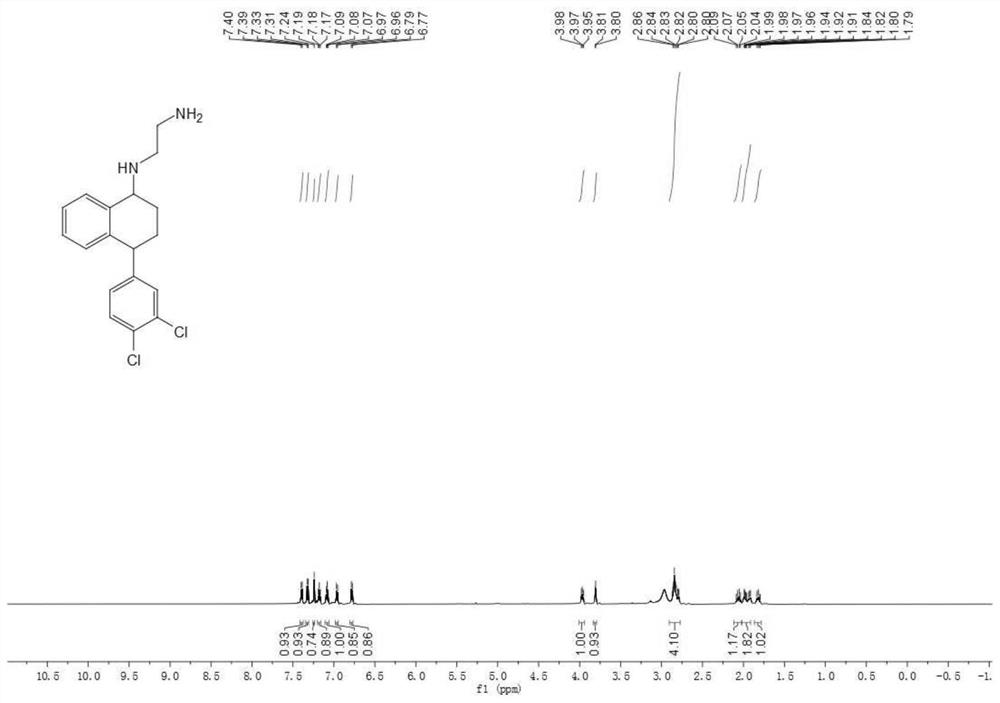
[0069] Synthesis of embodiment 3 numbering NO.22 sertraline derivatives
[0070] Put the weighed intermediate compound III (583.9mg (2mmol)) in a dry three-neck round-bottomed flask with a stirring bar, carry out anhydrous and anaerobic treatment, and then add Boc-protected ethylenediamine (0.65ml (4mmol) )) After stirring for 5 minutes, tetraisopropyl titanate (2ml (6.75mmol)) was added thereto and refluxed in an oil bath at 85°C for 2 hours. After 2 hours, it was cooled to room temperature and then cyanogen was added thereto. Sodium borohydride (314.2 mg (5 mmol)) was heated to 85 degrees Celsius and refluxed until the reaction was complete. After the reaction is complete, the reaction solution is cooled to room temperature, and then quenched by adding an appropriate amount of saturated sodium bicarbonate solution, then extracted with dichloromethane, dried with anhydrous sodium sulfate, suction filtered, and spin-dried to obtain Yellow oil; then separated by column chromat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com