Furan ring 2, 5-disubstituted-tetrahydroisoquinoline compound and preparation and application thereof
A tetrahydroisoquinoline and compound technology, which is applied in the fields of medicinal chemistry synthesis and pharmacotherapy, can solve the problems of affecting the pharmacokinetic properties of chemotherapeutic drugs, non-negligible toxicity of small molecules of inhibitors, increasing the toxic and side effects of drugs, and the like. Novel structure, good biological activity, and the effect of improving drug efficacy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
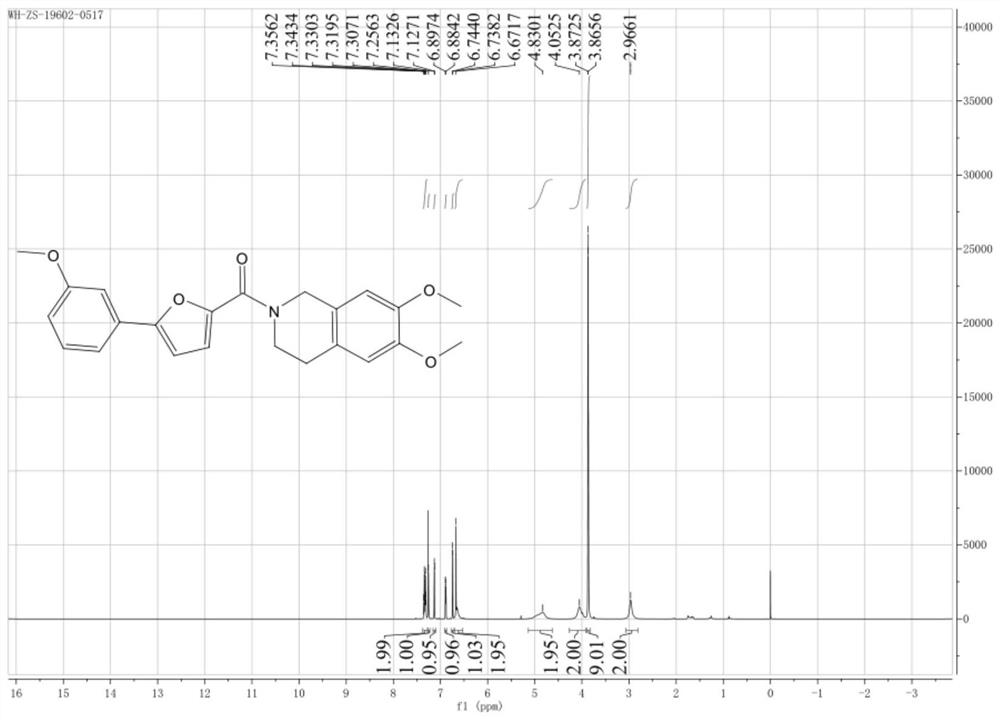
[0046] Example 1: 5-(2'-methoxyphenyl)-2-(2'-H-3,4-dihydro-6,7-methoxyisoquinoline)furan-2-carboxamide ( Ⅰ-2) Preparation
[0047]
[0048] (1) Add 5g (26.1mmol) of 5-bromo-2-furancarboxylic acid (II) and 10mL (156.2mmol) of dichloromethane into a 100mL three-necked flask equipped with a thermometer, stir and dissolve to form a clear solution. 7.5 g (63.0 mmol) of thionyl chloride was added with stirring. Raise the temperature to 60°C, react for 8 hours, and concentrate to dryness under reduced pressure with a rotary evaporator to remove excess solvent and unreacted thionyl chloride, and the obtained crude product 5.31g (25.6mmol) is 5-bromo-2-furyl chloride (Ⅲ), yield: 98%. for the next reaction.
[0049] (2) In a 50mL three-necked flask equipped with a thermometer, add 6,7-dimethoxy-1,2,3,4-tetrahydroisoquinoline hydrochloride (7.5mmol) and 10mL (156.2mmol) dichloro methane, stirred and cooled to 0°C, and 15mL of 4mol / L sodium hydroxide aqueous solution was added drop...
Embodiment 2
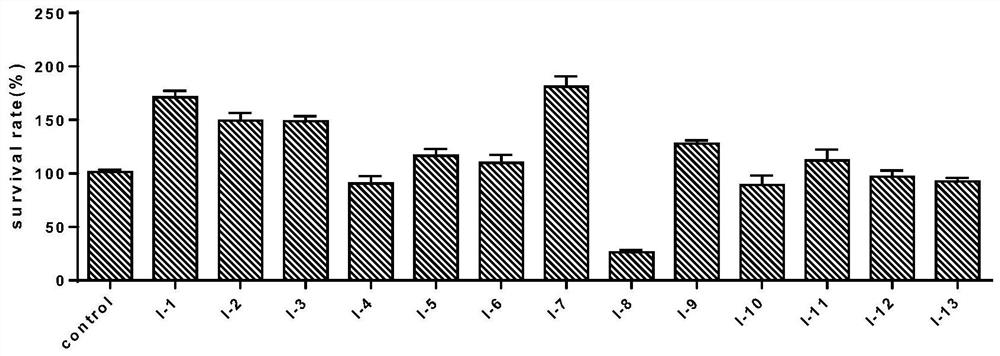
[0056] Cytotoxicity of 2,5-disubstituted-tetrahydroisoquinoline compounds containing furan ring shown in the formula I to MCF-7 / ADR
[0057] Cell line: MCF-7 / ADR (human breast cancer cell line resistant to doxorubicin, purchased from Nanjing Kaiji Biotechnology Development Co., Ltd.).
[0058] Sample test concentration: 5 μM, dissolved in RPMI 1640 medium containing 1% DMSO+10% fetal bovine serum and added.
[0059] Test method: MTT (tetramethyl azolium salt) method was used to test the cell proliferation activity of the compound.
[0060] MCF-7 / ADR cells were incubated with RPMI 1640 medium (purchased from Gbico) containing 10% calf serum at 37°C, 5% CO 2 cultured under saturated humidity conditions. Cells in the logarithmic growth phase were taken at 1×10 5 / mL density in 96-well culture plate, 100 μL per well at 37 ° C, 5% CO 2 Cultured under the condition of saturated humidity, divided into blank control group and test compound group. Different test compounds prepared...
Embodiment 3
[0063] Example 3. Research on the multidrug resistance reversal activity of 2,5-disubstituted-tetrahydroisoquinolines containing furan rings represented by formula I on MCF-7 / ADR cells.
[0064] Cell line: MCF-7 / ADR (human breast cancer cell line resistant to doxorubicin).
[0065] Sample test concentration: 5 μM, dissolved in RPMI 1640 medium containing 1% DMSO+10% fetal bovine serum and added.
[0066] Positive control drug: verapamil, dissolved in RPMI 1640 medium containing 1% DMSO + 10% fetal bovine serum by volume and then added.
[0067] Test method: The MTT (tetramethyl azolium salt) method was used to conduct the cell proliferation activity test of the compound and doxorubicin in combination.
[0068] MCF-7 / ADR cells were incubated with RPMI 1640 medium (purchased from Gbico) containing 10% calf serum at 37°C, 5% CO 2 cultured under saturated humidity conditions. Cells in the logarithmic growth phase were taken at 1×10 5 / mL density in 96-well culture plate, 100 μL ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com