Thiophene 2, 5-disubstituted-tetrahydroisoquinoline compound and preparation and application thereof
A technology of tetrahydroisoquinoline and tetrahydroisoquinoline hydrochloride, which is applied in the field of medicinal chemical synthesis and pharmacotherapeutics, can solve the problem of affecting the pharmacokinetic properties of chemotherapy drugs, the toxicity of small molecules of inhibitors cannot be ignored, and the increase Drug toxicity and side effects and other problems, to achieve good biological activity, high yield, improve the effect of drug efficacy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
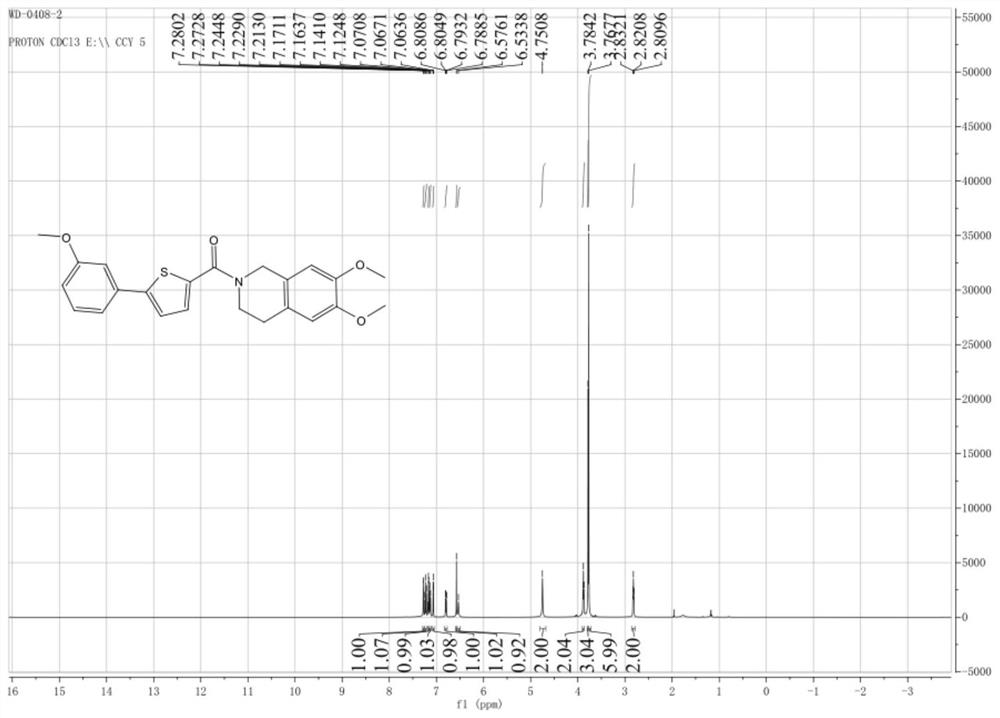
[0046] Example 1: 5-(3'-methoxyphenyl)-2-(2'-H-3,4-dihydro-6,7-methoxyisoquinoline)thiophene-2-carboxamide ( Ⅰ-2) Preparation
[0047]
[0048] (1) Add 5g (24.1mmol) of 5-bromo-2-thiophenecarboxylic acid (II) and 10mL (156.2mmol) of dichloromethane into a 100mL three-necked flask equipped with a thermometer, stir and dissolve to form a clear solution. 7.5 g (63.0 mmol) of thionyl chloride was added with stirring. Raise the temperature to 60°C, react for 8 hours and concentrate to dryness with a rotary evaporator to remove excess solvent and unreacted thionyl chloride, and the resulting crude product 5.37g (24.0mmol) is 5-bromo-2-thiophene chloride (Ⅲ ), yield: 98%. for the next reaction.
[0049] (2) Add 1.72g (7.5mmol) and 10mL (156.2mmol) of 6,7-dimethoxy-1,2,3,4-tetrahydroisoquinoline hydrochloride into a 50mL three-necked flask equipped with a thermometer Dichloromethane, stirred and cooled to 0°C, and 15 mL of 4mol / L sodium hydroxide aqueous solution was added drop...
Embodiment 2
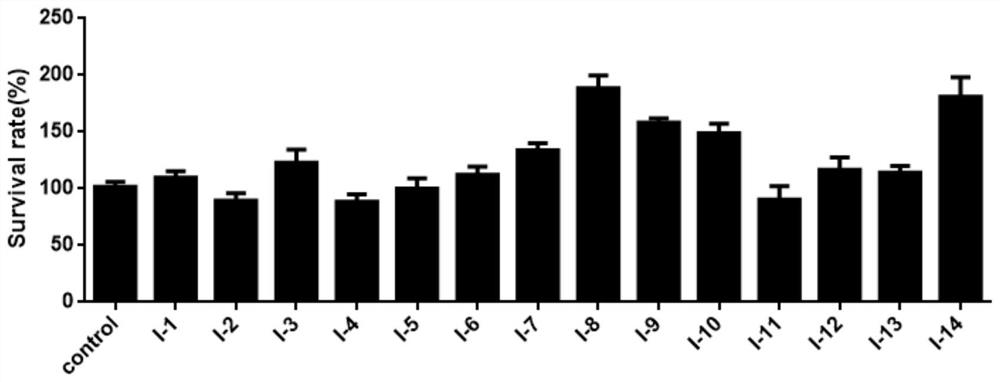
[0058] Cytotoxicity of thiophene-containing 2,5-disubstituted-tetrahydroisoquinoline compounds shown in embodiment 2, formula I to MCF-7 / ADR
[0059] Cell line: MCF-7 / ADR (human breast cancer cell line resistant to doxorubicin, obtained from Nanjing Kaiji Biotechnology Development Co., Ltd.).
[0060] Sample test concentration: 5 μM, dissolved in RPMI 1640 medium containing 1% DMSO+10% fetal bovine serum and added.
[0061] Test method: MTT (tetramethyl azolium salt) method was used to test the cell proliferation activity of the compound.
[0062] MCF-7 / ADR cells were incubated with RPMI 1640 medium (purchased from Gbico) containing 10% fetal bovine serum at 37°C, 5% CO 2 cultured under saturated humidity conditions. Cells in the logarithmic growth phase were taken at 1×10 5 / mL density in 96-well culture plate, 100 μL per well at 37 ° C, 5% CO 2 Cultured under the condition of saturated humidity, divided into blank control group and test compound group. Different compoun...
Embodiment 3
[0065] Example 3. Research on the multidrug resistance reversal activity of thiophene 2,5-disubstituted-tetrahydroisoquinoline compounds represented by formula (I) on MCF-7 / ADR cells.
[0066]Cell line: MCF-7 / ADR (human breast cancer cell line resistant to doxorubicin, obtained from Nanjing Kaiji Biotechnology Development Co., Ltd.).
[0067] Test compound test final concentration: 5 μM, dissolved in RPMI 1640 medium containing volume concentration 1% DMSO+10% calf serum and then added.
[0068] Positive control drug: verapamil, dissolved in RPMI 1640 medium containing volume concentration 1% DMSO+10% calf serum and then added.
[0069] Test method: The MTT (tetramethyl azolium salt) method was used to conduct the cell proliferation activity test of the compound and doxorubicin in combination.
[0070] MCF-7 / ADR cells were incubated with RPMI 1640 medium (purchased from Gbico) containing 10% calf serum at 37°C, 5% CO 2 cultured under saturated humidity conditions. Cells in ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com