New synthesis method of optically pure lactone
A technology of compounds and hydroxy esters, which is applied in the preparation of chiral drug sertraline chiral intermediates and ezetimibe chiral intermediates, and in the field of preparation of chiral lactones, which can solve the problem of large environmental pollution, high price and difficult availability , high toxicity and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-15
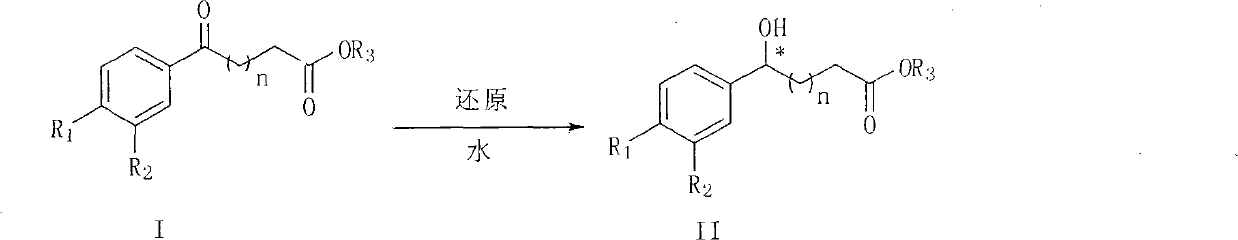
[0047] [RhCl 2 Cp*] 2 1.3 mg (0.002 mmol) of the chiral ligand L* (0.0044 mmol) was reacted with 1 ml of degassed water at 40° C. for 1 hour under the protection of argon. Add 95.2 mg (0.4 mmol) of ethyl 5-(4-fluorophenyl)-5-carbonyl butyrate and 208 mg (2 mmol) of sodium formate dihydrate, and react at 40°C for 4 hours under the protection of argon. Extract with diethyl ether 3x3 ml, dry over anhydrous sodium sulfate, remove the metal complex with a silica gel layer, and remove the solvent under reduced pressure to obtain a light brown oil. NMR determined the content of each component. The product represents ethyl 5-(4-fluorophenyl)-5-hydroxybutyrate and the substrate represents ethyl 5-(4-fluorophenyl)-5-oxobutyrate. e.e value determination method: chiral OD column, mobile phase: n-hexane / isopropanol=98 / 2, flow rate: 1mL / min -1 , detection wavelength: 254nm, t R =29.5,t S = 31.1. The e.e value of the product / substrate <50% on NMR was not measured.
[0048] Results of...
Embodiment 16
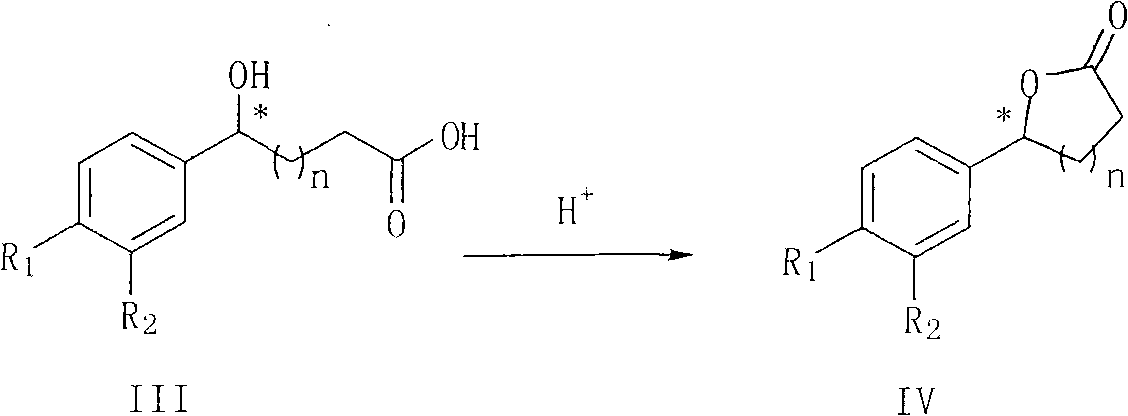
[0055] [RhCl 2 Cp*] 2 1.3 mg (0.002 mmol), 1.9 mg (0.0044 mmol) of (S,S,R)-Cs-DPEN and 1 ml of degassed water were reacted at 40°C for 1 hour under the protection of argon. Add 89.6 mg (0.4 mmol) of methyl 4-(4-fluorobenzoyl)butyrate and 208 mg (2 mmol) of sodium formate dihydrate, and react at 40° C. for 4 hours under the protection of argon. Add 0.5 ml of 6N sodium hydroxide solution, stir for 0.5 hours, add 1N hydrochloric acid at room temperature to adjust the pH value to about 2, extract with ethyl acetate 3x10 ml, dry over anhydrous sodium sulfate, remove metal complexes with silica gel layer, and reduce pressure Remove solvent. Then 1 ml of 1N hydrochloric acid and 5 ml of dichloromethane were added and stirred at 25°C for 10 hours, monitored by TLC (ethyl acetate:petroleum ether=1:1) until the reaction was complete. The organic layer was separated, and the aqueous phase was extracted with 3×5 milliliters of dichloromethane, the organic layers were combined, dried ov...
Embodiment 17
[0057] [RhCl 2 Cp*] 2 1.3 mg (0.002 mmol), 1.9 mg (0.0044 mmol) of (S,S,R)-Cs-DPEN and 1 ml of degassed water were reacted at 40°C for 1 hour under the protection of argon. Add 95.2 mg (0.4 mmol) of ethyl 4-(4-fluorobenzoyl)butyrate and 208 mg (2 mmol) of sodium formate dihydrate, and react at 40° C. for 4 hours under the protection of argon. Add 0.5 ml of 6N sodium hydroxide solution, stir for 0.5 hours, add 1N hydrochloric acid at room temperature to adjust the pH value to about 2, extract with ethyl acetate 3x10 ml, dry over anhydrous sodium sulfate, remove metal complexes with silica gel layer, and reduce pressure Remove solvent. Then 1 ml of 1N hydrochloric acid and 5 ml of dichloromethane were added and stirred at 25°C for 10 hours, monitored by TLC (ethyl acetate:petroleum ether=1:1) until the reaction was complete. The organic layer was separated, and the aqueous phase was extracted with 3×5 milliliters of dichloromethane, the organic layers were combined, dried ove...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com