Olanzapine orally-disintegrating tablet preparation and preparation method thereof
A technology for olanzapine orally disintegrating tablets, applied in the field of olanzapine orally disintegrating tablets and their preparation, can solve the problems of being unsuitable for long-term storage, transportation and use, unfavorable promotion and application, industrial large-scale production of splits, etc., and achieves improved biological utilization. The effect of speed, drug dissolution, and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
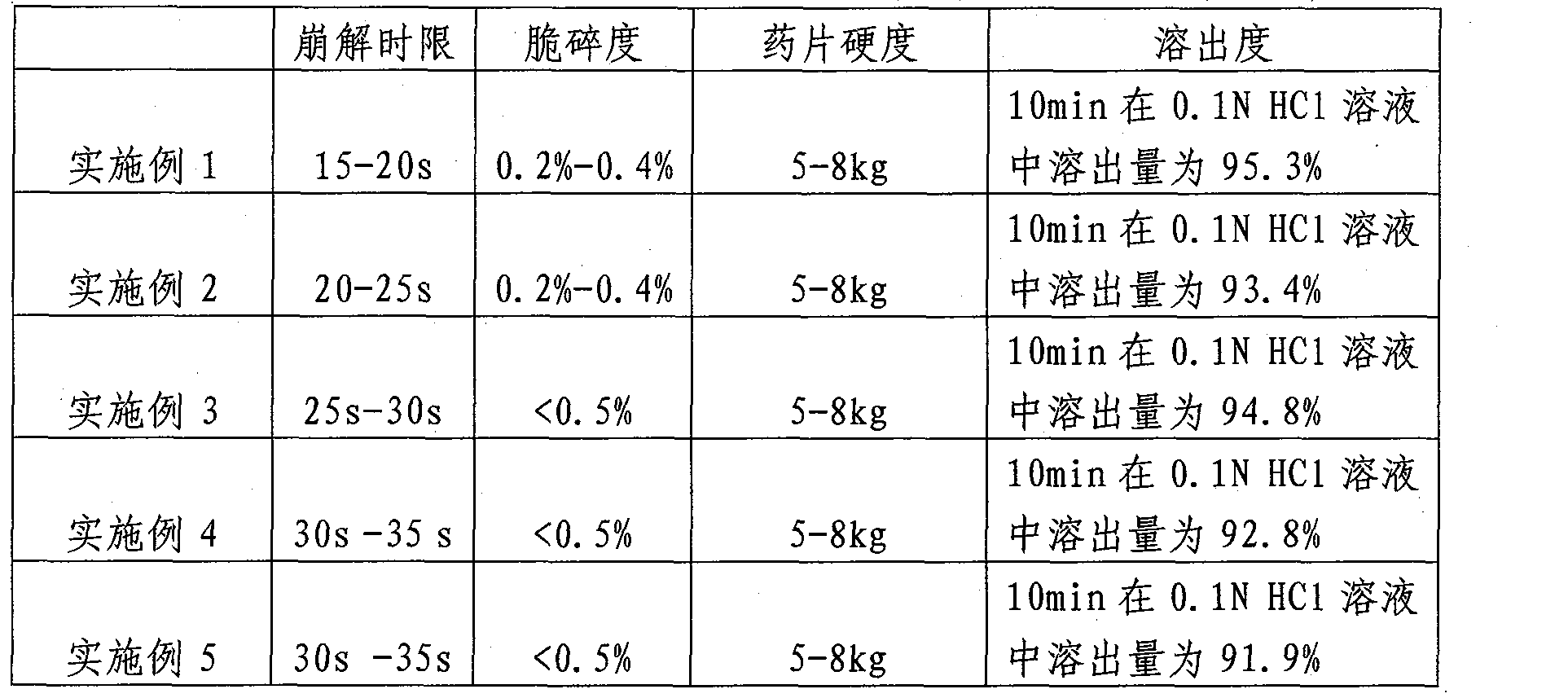
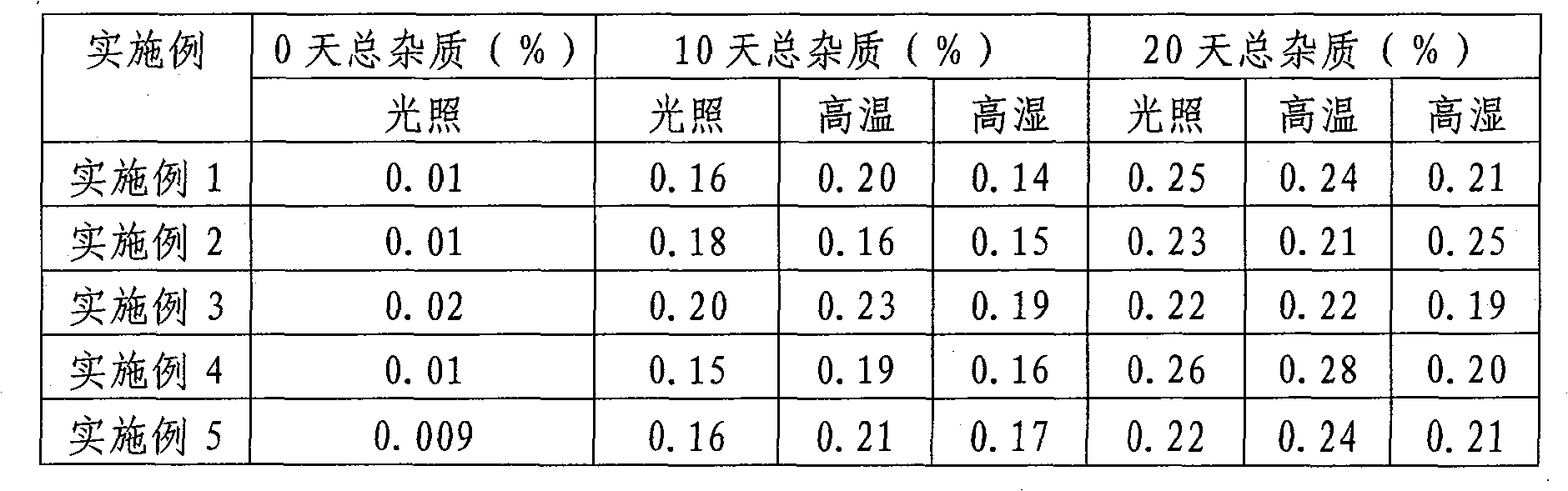
Examples
Embodiment 1
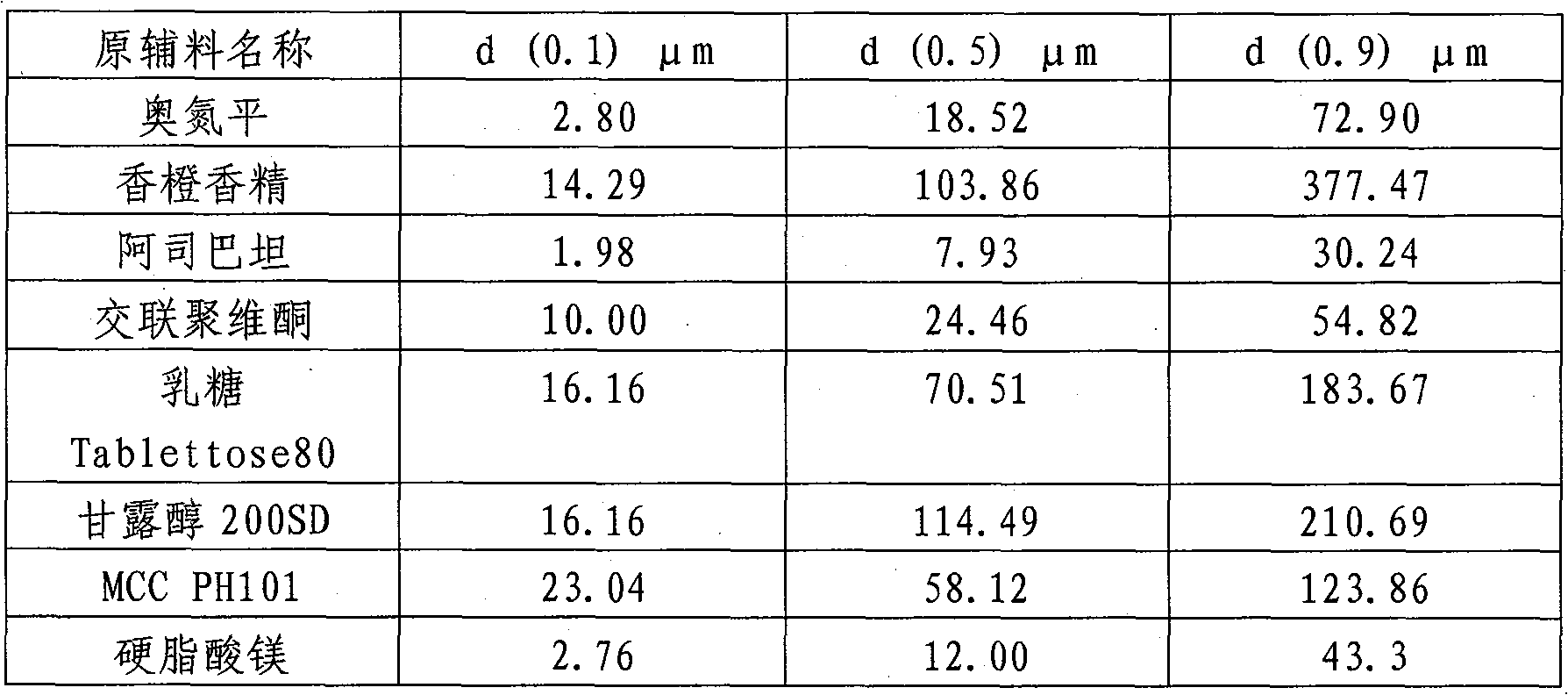
[0047] The prescription is:
[0048] Olanzapine (ADP) 5%
[0049] Mannitol 200SD 47.1%
[0050] Lactose (Tabllettose 80) 40.3%
[0051] Cross-linked polyvinylpyrrolidone (PVPP) 5%
[0052] Aspartan 0.6%
[0053] Orange flavor 0.5%
[0054] Magnesium Stearate 1.5%
[0055] Preparation: This dosage form can be produced by using conventional tablet pharmaceutical equipment and prepared by direct tableting process. The specific preparation method is as follows: grind the essence, aspartame and main drug separately and pass through an 80-mesh sieve, and the essence, aspartame and The main ingredients are mixed evenly; the cross-linked polyvinylpyrrolidone is passed through a 100-mesh sieve, and the mannitol and lactose are respectively passed through a 40-mesh sieve, respectively weighed according to the amount, and then added to the main ingredients mixed with essence and aspartam and mixed evenly, and then add the prescribed amount of Magnesium stearate, sieved and mixed, a...
Embodiment 2
[0057] The prescription is:
[0058] Olanzapine (ADP) 6.4%
[0059] Mannitol 200SD 38%
[0060] Lactose (Tab lettose 80) 38%
[0061] Microcrystalline Cellulose (MCC PH101) 10%
[0062] Cross-linked polyvinylpyrrolidone (PVPP) 5%
[0063] Aspartan 0.6%
[0064] Orange flavor 0.5%
[0065] Magnesium Stearate 1.5%
[0066] Preparation: This dosage form can be produced by using conventional tablet pharmaceutical equipment and prepared by direct tableting process. The specific preparation method is as follows: grind the essence, aspartame and main drug separately and pass through an 80-mesh sieve, and the essence, aspartame and The main ingredients are mixed evenly; the cross-linked polyvinylpyrrolidone is passed through a 100-mesh sieve, and the mannitol, lactose and microcrystalline cellulose are respectively passed through a 40-mesh sieve. Mix well, then add the prescribed amount of magnesium stearate, sieve and mix, and detect the intermediate content. After determinin...
Embodiment 3
[0068] The prescription is:
[0069] Olanzapine (ADP) 8%
[0070] Mannitol 200SD 38.44%
[0071] Lactose (Tabllettose 80) 32.96%
[0072] Microcrystalline Cellulose (MCC PH101) 8%
[0073] Low-substituted hydroxypropyl cellulose (L-HPC) 8%
[0074] Cross-linked polyvinylpyrrolidone (PVPP) 2%
[0075] Aspartan 0.6%
[0076] Orange flavor 0.5%
[0077] Magnesium Stearate 1.5%
[0078] Preparation: The dosage form can be produced by using conventional tablet pharmaceutical equipment and prepared by dry granulation and tabletting technology. The specific preparation method is as follows: the main drug, orange essence, aspartame, mannitol, lactose, microcrystalline cellulose, Low-substituted hydroxypropyl cellulose and cross-linked polyvinylpyrrolidone were respectively passed through a 100-mesh sieve, weighed according to the amount and added in sequence, sieved and mixed, pressed into tablets, and passed through a 18-mesh sieve for dry granulation. Then add the magnesium ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Sheet weight | aaaaa | aaaaa |
| Hardness | aaaaa | aaaaa |
| Hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com