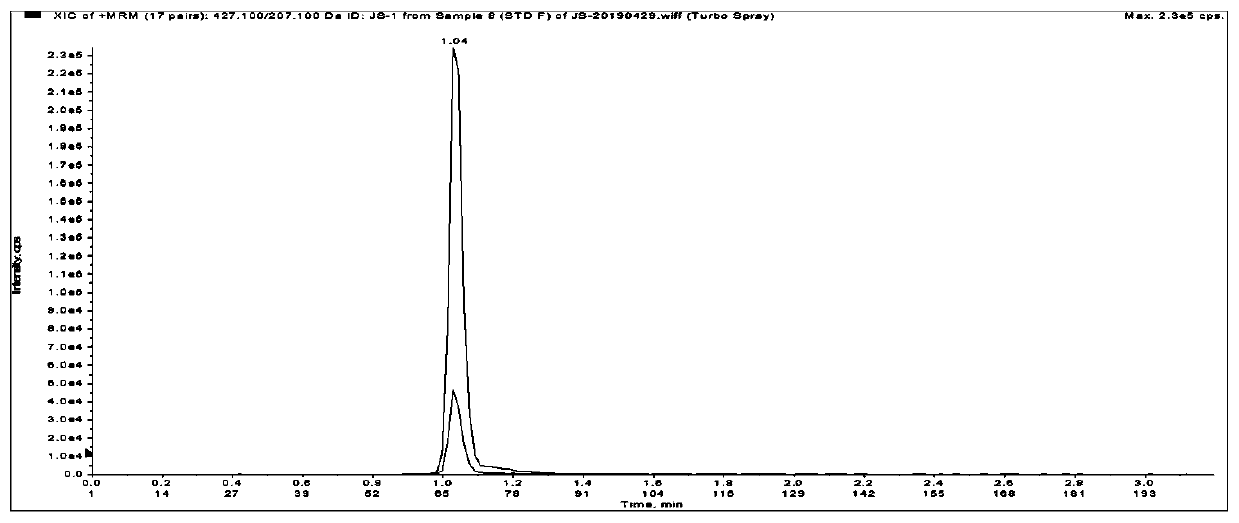
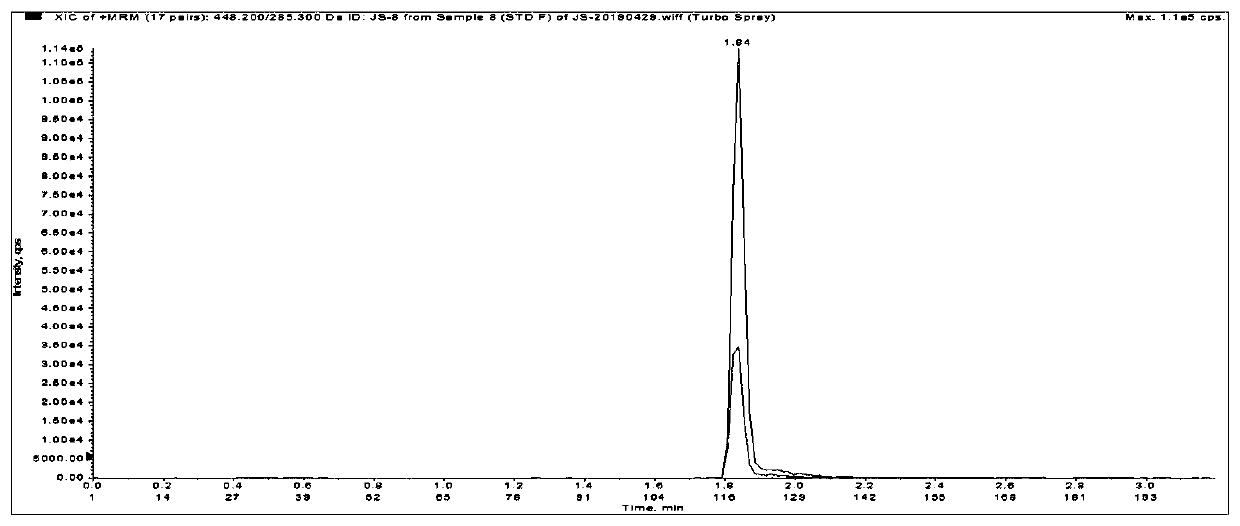
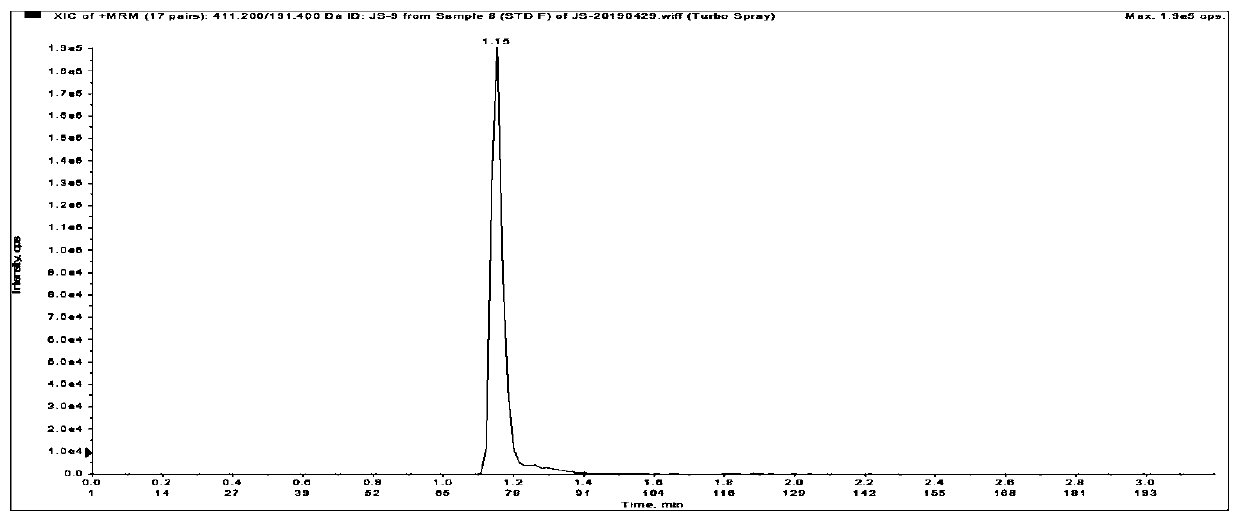
Method for detecting liquid quality of antipsychotic drug in serum or plasma
A detection method and technology of psychotropic drugs, applied in the field of medical inspection and analysis, to achieve high accuracy of results, accurate qualitative and quantitative results, simple and reliable operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] The recovery rate experiment of 8 kinds of antipsychotic drugs in serum:
[0041] 1. Materials and reagents
[0042] Chromatographic column: Kinetex C18 5μm, 50mm×3.0mm
[0043] Risperidone, clozapine, haloperidol, quetiapine and ziprasidone were purchased from China Institute for Food and Drug Control; paliperidone, quetiapine fumarate-d8 and ziprasidone-d8 were purchased From SIGMA; risperidone-d4, aripiprazole-d8, aripiprazole, olanzapine, olanzapine-d8, clozapine-d4, haloperidol-d4 and -hydroxyrisperidone- d4 was purchased from Cerilliant; methanol, acetonitrile, isopropanol, formic acid, ammonium formate (chromatographically pure), ammonia (chromatographically pure); ultrapure water: prepared by a Mili-Q ultrapure water machine.
[0044] 2. Instruments and equipment
[0045] High performance liquid chromatography-tandem mass spectrometer, equipped with electrospray ionization (ESI) ionization source (TRIPLE QUAD 4500MD, AB SCIEX, USA), liquid chromatographic separation mod...
Embodiment 2
[0070] 1. Materials and reagents
[0071] The difference from Example 1 is that the protein precipitation agent used in the pretreatment process is reconfigured according to the ratio of methanol: pure water (3:1);
[0072] 2. Apparatus and equipment
[0073] High performance liquid chromatography-tandem mass spectrometer, equipped with electrospray ionization (ESI) ionization source (API 3200MD, AB SCIEX, USA), liquid chromatographic separation mode is reversed phase chromatography, and the detector is triple quadrupole tandem mass spectrometry; One part electronic analytical balance; SPE solid phase extraction device.
[0074] 3. Matrix effect of 8 antipsychotic drugs in serum (without solid phase extraction pretreatment):
[0075] The pre-processing steps for detecting the matrix effect of 8 antipsychotic drugs by high performance liquid chromatography-tandem mass spectrometry are as follows:
[0076] 1) Serum extract + standard: Take 100μL of blank serum into a 1.5mL centrifuge tube...
Embodiment 3
[0105] Compare the extraction of 8 antipsychotic drugs with different types of commercial solid phase extraction columns C18, C18Phe and specific purification solid phase extraction columns.
[0106] 1. Materials and reagents
[0107] Commercial solid phase extraction column C18, C18Phe, specific purification packing.
[0108] 2. Apparatus and equipment
[0109] Same as Example 2.
[0110] 3. High performance liquid chromatography-tandem mass spectrometry detection of different types of solid phase extraction columns for the pretreatment of 8 antipsychotic drugs, the steps are:
[0111] 1) Add 100μL of human serum + 5μL of quality control (M) into a 1.5mL centrifuge tube, add 300μL of protein precipitation agent (acetonitrile: water = 3:1), vortex to mix, centrifuge at low temperature and high speed at 13500rpm for 5min, take the supernatant for use , In triplicate;
[0112] 2) Packing solid phase extraction column: Packing C18, C18Phe and the specific purification material of the presen...
PUM
| Property | Measurement | Unit |
|---|---|---|
| volume | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com