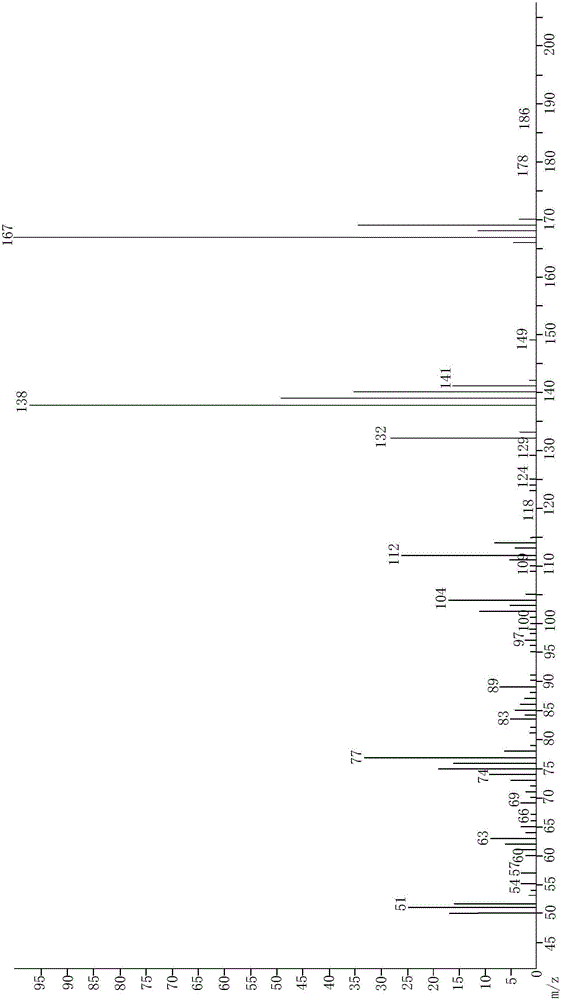
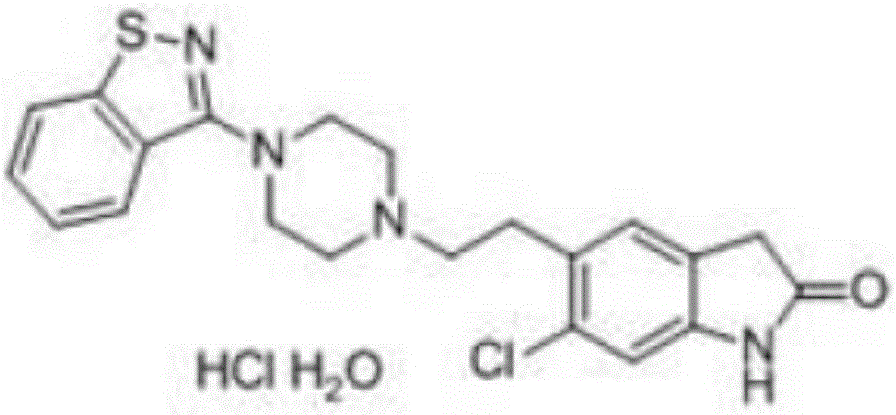
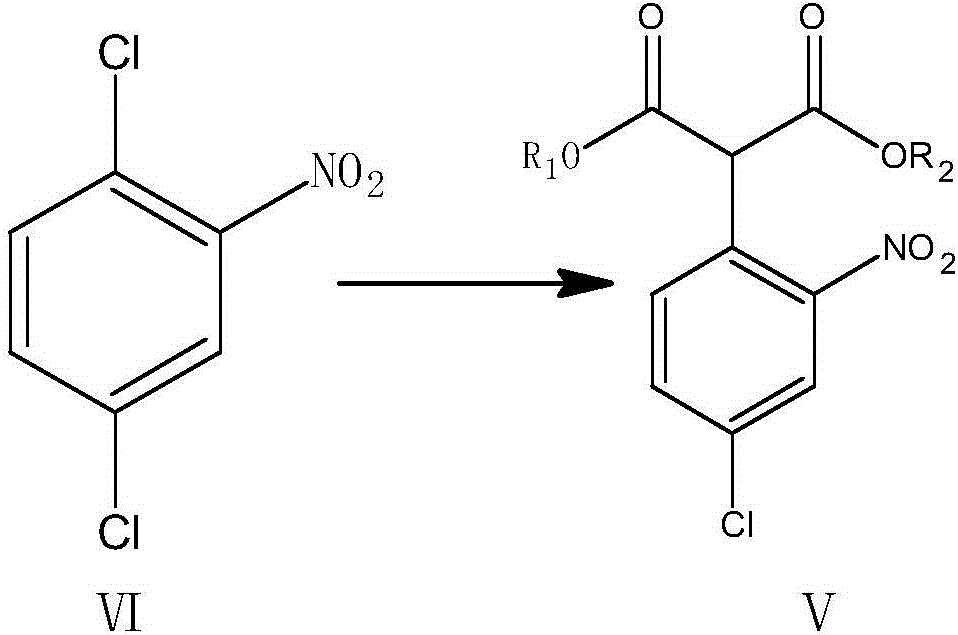
Preparation method of ziprasidone intermediate
A technology of chiprasidone and intermediates, which is applied in the field of synthesis of pharmaceutical intermediates, can solve the problems of unfavorable industrial promotion, long synthetic route, dangerous operation, etc., and achieve simple and easy process, mild deacidification process, and safe production Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] In the present embodiment, the preparation method of 6-chloro-2-indolinone, an intermediate of ziprasidone, comprises the following steps:
[0042] (1) Add 2500mL of acetone to the three-necked flask, add 200g of dimethyl malonate, 240g of 2,5-dichloronitrobenzene, 420g of potassium carbonate powder, and 10g of tetrabutylammonium bromide under the action of stirring. React at ~60°C for 20 hours, and TLC (PE:EA=3:1) detects that the raw materials disappear; cool down to room temperature, filter off the solid acid-binding agent, and recover acetone under reduced pressure to obtain the oily 2-(4-chloro-2-nitrate dimethylphenyl)malonate;
[0043] (2) Add 1000 mL of ethanol and 1000 g of 2N NaOH to the product of step (1) under stirring, react at 70 to 80° C. for 6 hours, until the raw materials are detected by TLC (PE:EA=3:1), and 2-(4- Chloro-2-nitrophenyl) malonate disodium salt;
[0044] (3) The reaction system of step (2) is cooled to 0-40 DEG C, and concentrated hydr...
Embodiment 2
[0049]In the present embodiment, the preparation method of 6-chloro-2-indolinone, an intermediate of ziprasidone, comprises the following steps:
[0050] (1) Add 2500mL butanone into the three-necked flask, add 200g of dimethyl malonate, 240g of 2,5-dichloronitrobenzene, 380g of anhydrous sodium carbonate powder, benzyl triethyl chloride under stirring Ammonium 10g, react in a water bath at 55-60°C for 20h, TLC (PE:EA=3:1) detects that the raw materials disappear; cool down to room temperature, filter off the solid acid-binding agent, recover butanone under reduced pressure, and obtain the oily 2-(4 -Dimethyl chloro-2-nitrophenyl) malonate;
[0051] (2) Add 1500 mL of ethanol and 1200 g of 2N KOH to the product of step (1) under stirring, and react at 70 to 80° C. for 6 hours. TLC (PE:EA=3:1) detects that the raw materials disappear, and 2-(4- Chloro-2-nitrophenyl) malonate dipotassium salt;
[0052] (3) The reaction system of step (2) is cooled to 0-40 DEG C, and concentrat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com