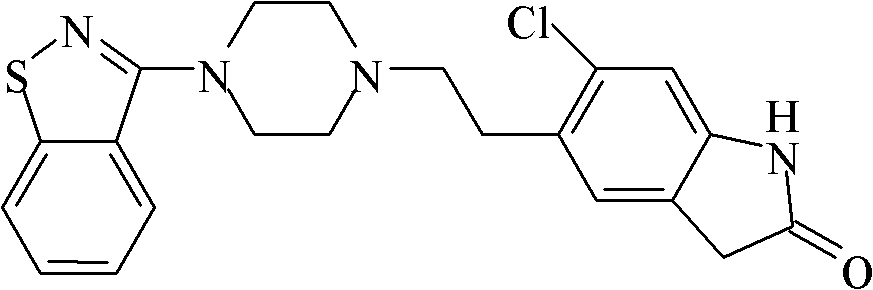
Method for preparing ziprasidone
A ziprasidone and water-soluble technology, applied in the field of ziprasidone preparation, can solve the problems of cumbersome and low-efficiency production process, unfavorable scale production, long reaction time and the like, and achieves increased acid binding effect, increased solubility, impurities low content effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Add 320mL of acetone into a 500ml three-necked flask, start stirring, and put in 20.0g (86.9mmol) of 5-(2-chloroethyl)-6-chloro-1,3-dihydro-indol-2-(2H)-one , 3-(1-piperazinyl)-1,2-benzisothiazole hydrochloride 23.3g (91.1mmol), then add 80mL 20%K 2 CO 3 Heat the aqueous solution to 60-70°C and keep it warm for 5 hours. After the reaction is detected by TLC, cool down to 30°C, add 480mL of purified water, stir at 20°C to 30°C for 30 minutes, filter with suction, rinse the filter cake with 40mL of purified water, and The solid material was dried in a vacuum oven at 60° C. for 4 hours to obtain 32.0 g of ziprasidone, with a yield of 89.2%, and a purity of 99.0% by HPLC.
Embodiment 2
[0025] Add 210mL tetrahydrofuran into a 500ml three-necked flask, start stirring, and add 15.0g (65.2mmol) of 5-(2-chloroethyl)-6-chloro-1,3-dihydro-indol-2-(2H)-one , 3-(1-piperazinyl)-1,2-benzisothiazole hydrochloride 17.0g (66.5mmol), then add 90mL 20%Na 2 CO 3 As an aqueous solution, heat up to 60-70°C, keep it warm for 5 hours, cool down to 30°C after the reaction is detected by TLC, add 225mL of purified water, keep stirring for 45 minutes after cooling down, filter with suction, and rinse the filter cake with 30mL of purified water. The solid material was dried in a vacuum oven at 60° C. for 4 hours to obtain 24.4 g of ziprasidone with a yield of 90.6% and a purity of 99.1% by HPLC.
Embodiment 3
[0027] Add 280mL of N,N-dimethylformamide into a 500ml three-necked flask, start stirring, and add 5-(2-chloroethyl)-6-chloro-1,3-dihydro-indole-2-(2H) - Ketone 20.0g (86.9mmol), 3-(1-piperazinyl)-1,2-benzisothiazole hydrochloride 23.3g (91.1mmol), then add 120mL 20%Na 2 CO 3 aqueous solution. Raise the temperature to 60-70°C and react for 4 hours. After the reaction is detected by TLC, cool down to 30°C, add 280mL of purified water, stir at 20°C to 30°C for 30 minutes, filter with suction, rinse the filter cake with 40mL of purified water, and remove the solid material Put it into a vacuum oven at 60° C. and dry for 4 hours to obtain 32.8 g of ziprasidone with a yield of 91.4% and a purity of 99.3% by HPLC.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com