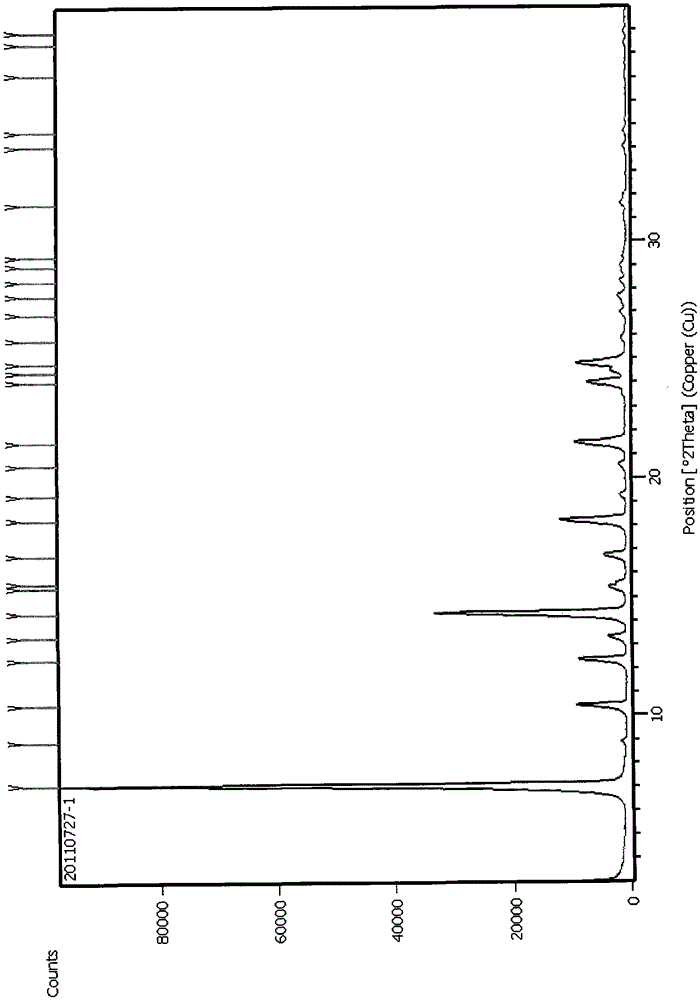
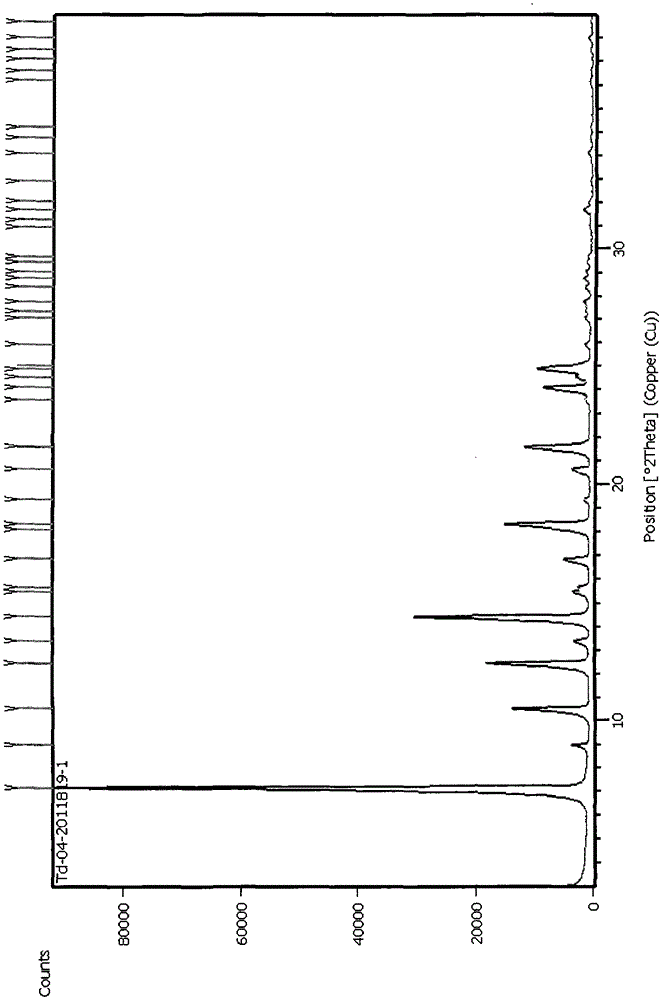
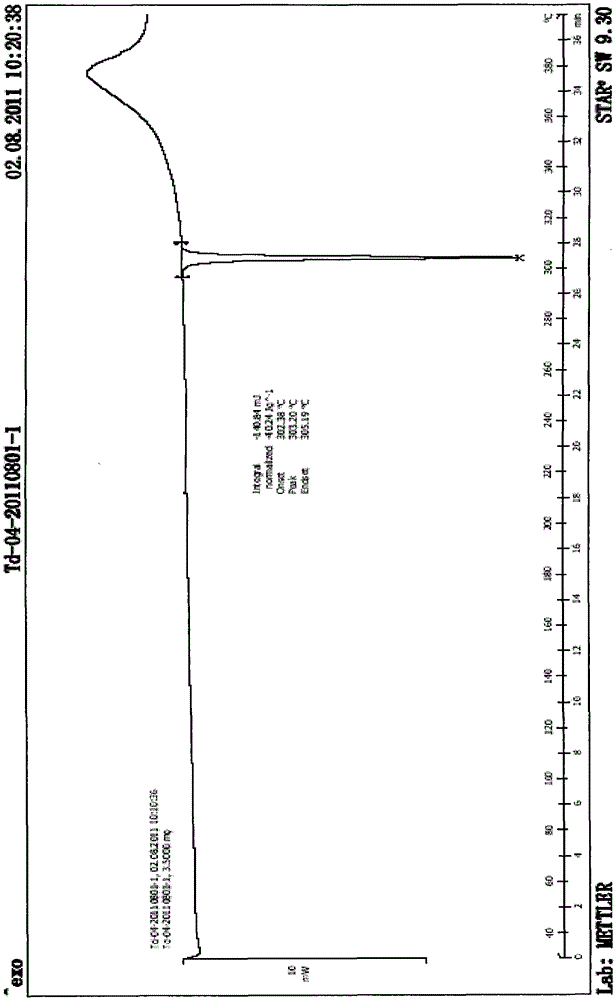
A method for preparing tadala amorphous form a
A technology of tadalafil and amorphous form, which is applied in the field of preparation of tadalafil amorphous form A, can solve the problems that it is difficult to fully reduce the residual acetic acid solvent and the cumbersome processing process, and achieve low residual acetic acid solvent and simplify drying Effect of reduction in process and usage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Example 1: Preparation of Tadala Amorphous Form A by Using Methanol / Acetic Acid as Solvent
[0038] In a 100ml round bottom flask, add 1.0g of tadalafil and 45ml of methanol / acetic acid mixed solvent (methanol:acetic acid volume ratio 0.8:1). Heated to 83-85°C to obtain a clear solution of tadalafil, and continued to stir for 30-60 minutes. The solution was naturally cooled to room temperature (25-30°C) for crystallization. The solution was further cooled to -5-0°C and stirred for 30-60 minutes for further crystallization. Filter, wash with a small amount of methanol, and drain the solvent to obtain a wet product of Tadalamorph Amorphous Form A. Dry the wet product of tadala amorphous form A at 50-55° C. to obtain 0.5 g of tadala amorphous form A dry product.
Embodiment 2
[0039] Example 2: Preparation of Tadala Amorphous Form A by Using Methanol / Acetic Acid as Solvent
[0040] In a 100ml round bottom flask, add 1.0g of tadalafil and 27ml of methanol / acetic acid mixed solvent (methanol:acetic acid volume ratio 0.6:1). Heated to 83-85°C to obtain a clear solution of tadalafil, and continued to stir for 30-60 minutes. The solution was naturally cooled to room temperature (25-30°C) for crystallization. The solution was further cooled to -5-0°C and stirred for 30-60 minutes for further crystallization. Filter, wash with a small amount of methanol, and drain the solvent to obtain a wet product of Tadalamorph Amorphous Form A. Dry the wet product of tadala amorphous form A at 50-55° C. to obtain 0.5 g of tadala amorphous form A dry product.
Embodiment 3
[0041] Example 3: Preparation of Tadala Amorphous Form A by Using Methanol / Acetic Acid as Solvent
[0042] In a 100ml round bottom flask, add 1.0g of tadalafil and 17.5ml of methanol / acetic acid mixed solvent (methanol:acetic acid volume ratio 0.4:1). Heated to 83-85°C to obtain a clear solution of tadalafil, and continued to stir for 30-60 minutes. The solution was naturally cooled to room temperature (25-30°C) for crystallization. The solution was further cooled to -5-0°C and stirred for 30-60 minutes for further crystallization. Filter, wash with a small amount of methanol, and drain the solvent to obtain a wet product of Tadalamorph Amorphous Form A. Dry the wet product of tadala amorphous form A at 50-55° C. to obtain 0.5 g of tadala amorphous form A dry product.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com