Preparation method of pharmaceutical composition preparation treating apoplexy sequelae
A technology for stroke sequelae and composition, which is applied in the direction of drug combination, pharmaceutical formula, medical preparations containing active ingredients, etc., which can solve the problems of slow release of active ingredients of raw materials, affecting the absorption and utilization of active ingredients of drugs, etc., to achieve onset of effect Fast, improved drug compliance, and improved curative effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-4
[0024] The proportioning by weight of the crude drug of embodiment 1-4 pharmaceutical composition is as follows:
[0025] 17.3 parts of pearl, 2.2 parts of geranium, 8.7 parts of saffron, 9.7 parts of clove, 8.7 parts of nutmeg, 8.7 parts of cardamom, 6.5 parts of grass fruit, 8.7 parts of sandalwood, 21.6 parts of rosewood, 17.3 parts of agarwood, 28.1 parts of myrobalan 17.3 parts of myrobalan, 21.6 parts of emblica, 21.6 parts of woody fragrance, 17.3 parts of cinnamon, 8.7 parts of longan, 10.8 parts of crab, 8.7 parts of golden stone, 5.4 parts of parsley, 2.2 parts of artificial bezoar, and 2.2 parts of musk 6.5 parts of jujube, 6.5 parts of rhododendron, 13.0 parts of Corydalis cypress, 43.3 parts of rabbit ear grass, 4.3 parts of iron powder (manufactured), 17.3 parts of mallow fruit, 13.0 parts of licorice, 5.4 parts of black seed .
[0026] Zhenlong Xingnao Capsules, which were originally commercially available for comparison, are products of Qinghai Jinhe Tibetan M...
Embodiment 1
[0027] Embodiment 1, the preparation of a kind of pharmaceutical composition capsule for the treatment of stroke sequelae
[0028] (1) Extraction and inclusion of volatile oil: according to the composition ratio of raw materials, take 435g of longan longan, 485g of clove, 435g of nutmeg, 435g of cardamom, 325g of grass fruit, 270g of parsley, 435g of sandalwood, 270g of black cumin, and 865g of agarwood , mallow fruit 865g, cinnamon 865g, woody 1080g, red sandalwood 1080g, strong rhododendron 325g, a total of 14 medicinal materials are mixed, and water is added to 6 times the total weight of the 14 medicinal materials, and the volatile oil is extracted by steam distillation for 4 hours. Collect volatile oil to obtain volatile oil, filter the medicinal solution to obtain extract A and medicinal residue A;
[0029]The obtained volatile oil was added to 4% by weight and volume percentage of β-cyclodextrin saturated aqueous solution, the volume-to-weight ratio of volatile oil to β...
experiment example 1
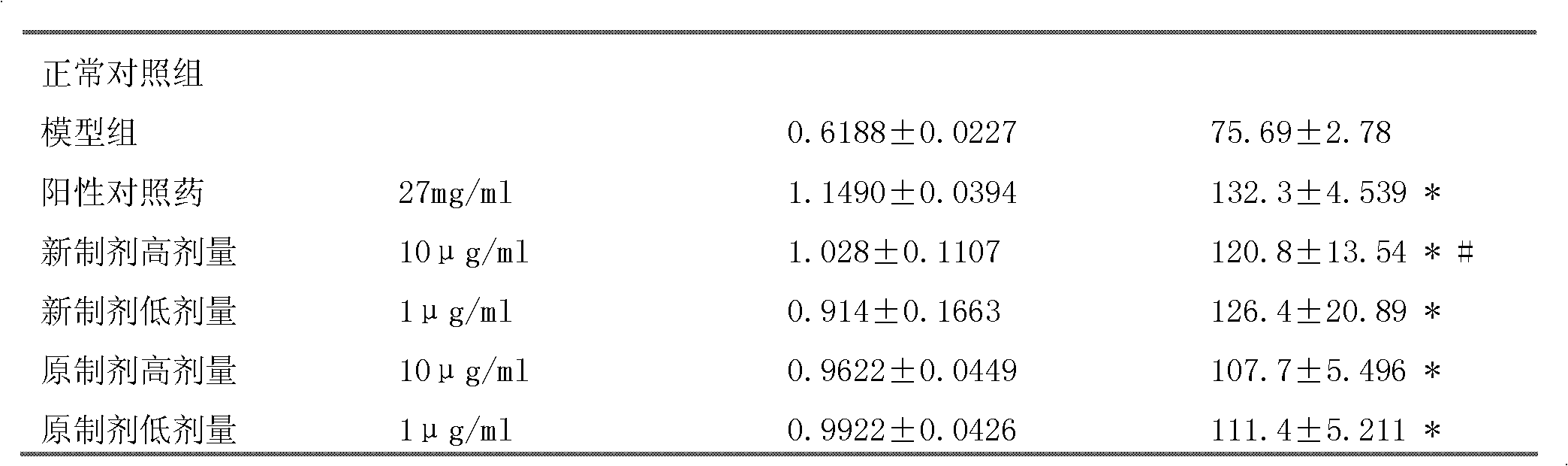
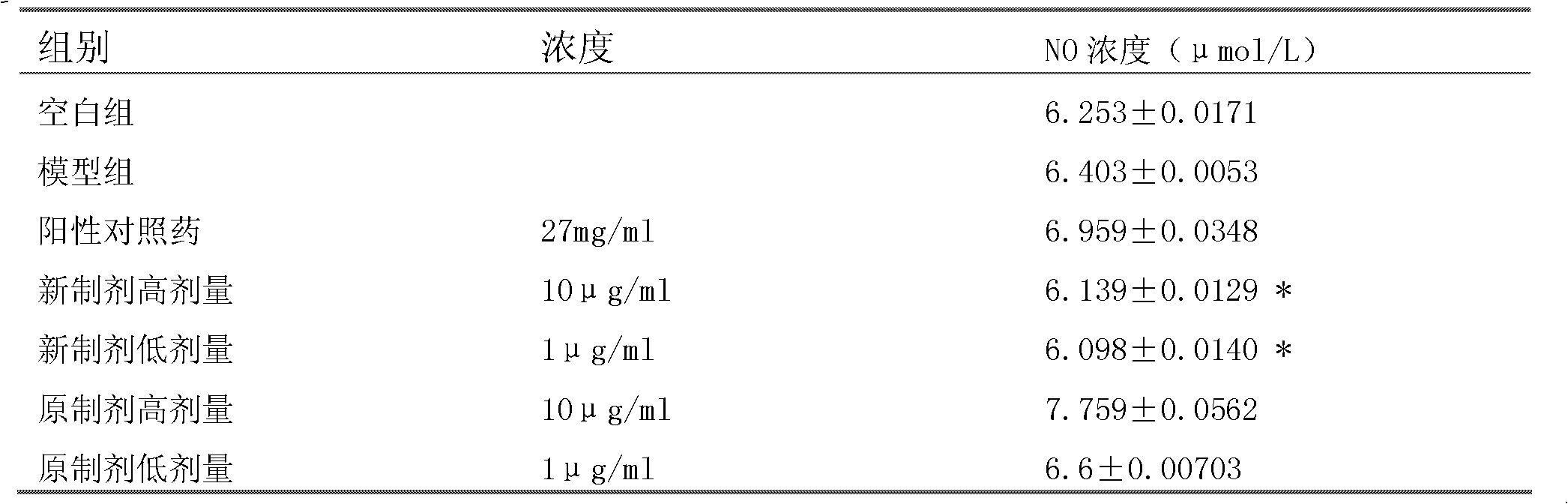
[0034] Experimental example 1. The new preparation of the above-mentioned embodiment 1, the original preparation of Zhenlong Xinnao Capsule and the positive control drug were used for the comparison experiment of nerve cell damage protection
[0035] 1. Experimental materials:
[0036] 1. The new preparation of Zhenlong Xinnao Capsule: the preparation Zhenlong Xinnao Capsule of Example 1;
[0037] 2. The original preparation of Zhenlong Xinnao Capsules (hereinafter referred to as the original preparation): Commercially available Zhenlong Xinnao Capsules (Qinghai Jinhe Tibetan Medicine Pharmaceutical Co., Ltd.), batch number: 20090908.
[0038] 3. Positive control drug: Compound Danshen Dripping Pills, Tianjin Tasly Pharmaceutical Co., Ltd., batch number: 100413.
[0039] 4. Cell line: PC12 cell line (source: Rattμs norvegicμs adrenal pheochromocytoma, provided by Nanjing KGI Biotechnology Development Co., Ltd.).
[0040] 5. Reagents: H-DMEM cell culture medium (Hyclone, Ther...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com