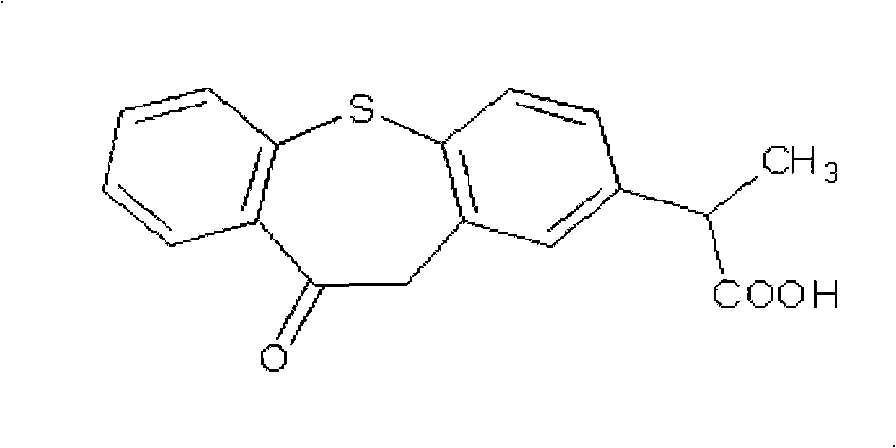
Zaltoprofen sustained-release preparation and preparation thereof
A slow-release preparation and slow-release technology, applied in anti-inflammatory agents, pill delivery, pharmaceutical formulations, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
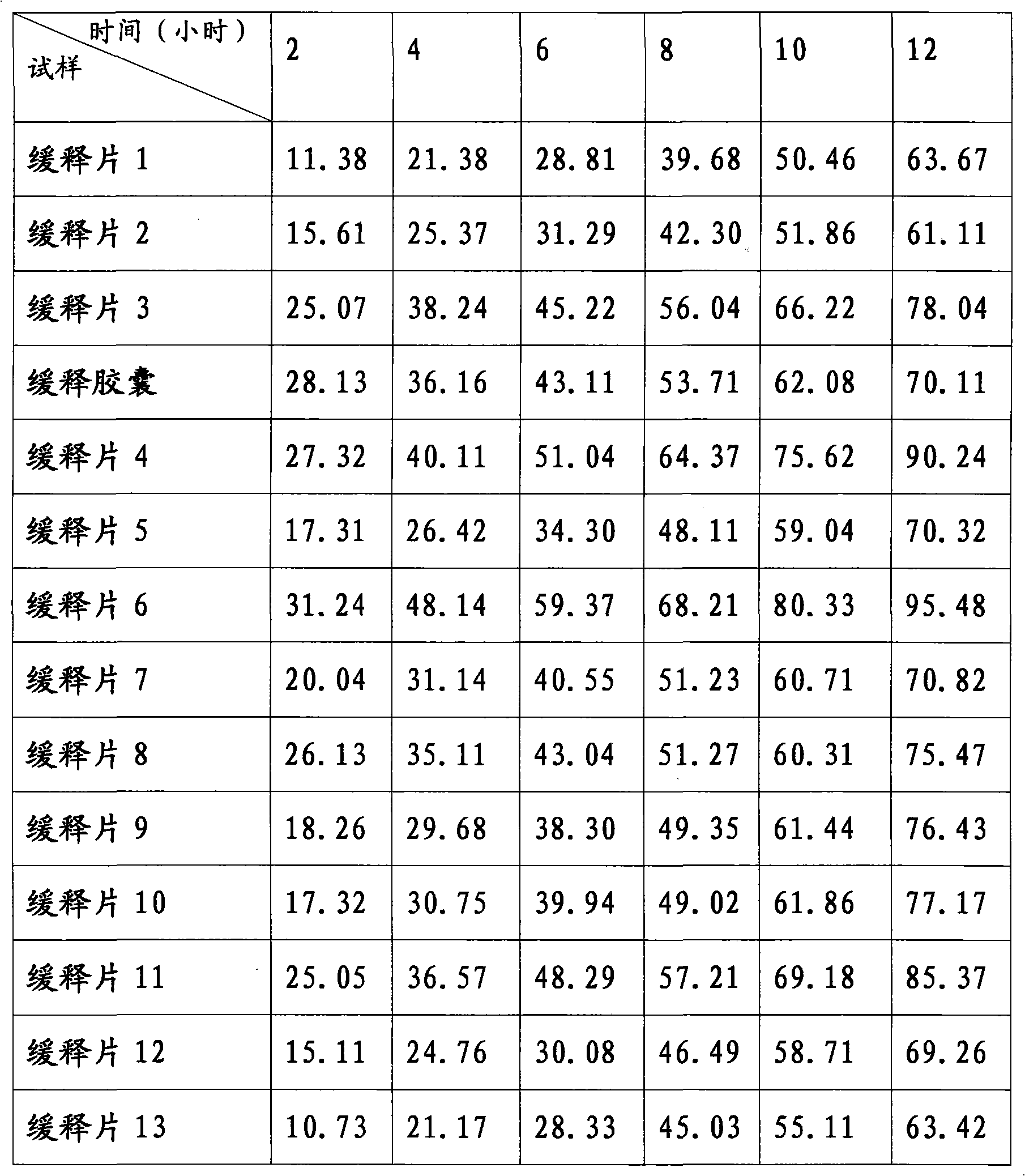
[0026] Example 1 Preparation of Zaltoprofen Sustained Release Tablet 1
[0027] Take Zaltobuprofen 120g and HPMC (K4M) 10g, pulverize them respectively, pass through a No. 9 sieve, put them into a mixing agitator, mix them evenly, use an appropriate amount of 5% PVPK30 ethanol as a binder, and mix them evenly with the above mixed powder Prepare soft materials, pass through a No. 2 sieve to make wet granules, and dry the obtained wet granules. The dried granules are mixed with an appropriate amount of magnesium stearate and micropowder silica gel, and compressed into tablets, 1000 tablets in total.
Embodiment 2
[0028] Example 2 Preparation of Zaltoprofen Sustained Release Tablet 2
[0029] Get Zaltobuprofen 120g, HPMC (K4M) 20g, HPMC (K15M) 20g, pulverize respectively, cross No. 9 sieves, put into mixing agitator, mix uniformly, be binding agent with appropriate amount of 5% PVPK30 ethanol liquid, Mix with the above mixed powder evenly to prepare a soft material, pass through a No. 2 sieve to make wet granules, and dry the obtained wet granules. The dried granules are mixed with an appropriate amount of magnesium stearate and micropowder silica gel, and compressed into tablets, 1000 tablets in total.
Embodiment 3
[0030] Example 3 Preparation of Zaltoprofen Sustained Release Tablet 3
[0031] Get Zaltobuprofen 80g, HPMC (K4M) 20g, HPMC (K100LV) 100g, pulverize respectively, cross No. 9 sieves, put into mixing agitator, mix uniformly, be binding agent with appropriate amount of 5% PVPK30 ethanol liquid, Mix with the above mixed powder evenly to prepare a soft material, pass through a No. 2 sieve to make wet granules, and dry the obtained wet granules. The dried granules are mixed with an appropriate amount of magnesium stearate and micropowder silica gel, and compressed into tablets, 1000 tablets in total.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com