Red cell membrane encapsulated polyester-type As2O3-supported nano particles and preparation method thereof
A technology of arsenic trioxide and red blood cell membranes, which is applied in the direction of medical formulas, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc., can solve problems such as digestive tract discomfort, complicated preparation process, liver and kidney function damage, and achieve Reduce blood concentration, significantly slow-release effect, reduce toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Example 1. Red blood cell membrane preparation (the prepared red blood cell membrane corresponds to an equal volume of whole blood):
[0035] (1) Rats were taken, weighed, anesthetized by intraperitoneal injection of chloral hydrate at 100g / 0.7mL, and whole blood was collected by cardiac puncture;
[0036] (2) Divide the blood into EP tubes containing 0.05mL heparin sodium, 1mL per tube, centrifuge at 3800r / min for 10min at 4°C to remove the supernatant and white blood cell layer, and dilute to 1mL with PBS solution, mix well, 3800r / min Min, 7min, wash twice at 4°C, and finally dilute to 1mL with PBS.
[0037](3) Divide each tube of the mixed solution obtained in the above step (2) into four EP tubes containing 0.01 mL of heparin sodium, then add 0.9 mL of EDTA solution, and mix well. Gently blow to break the wall, add 0.1mL 10 times concentration of PBS to mix, 13200r / min, 10min, 4°C centrifuge to wash, remove the supernatant, repeat this process more than three tim...
Embodiment 2
[0040] Embodiment 2. nanoparticle material, oil phase solvent screening
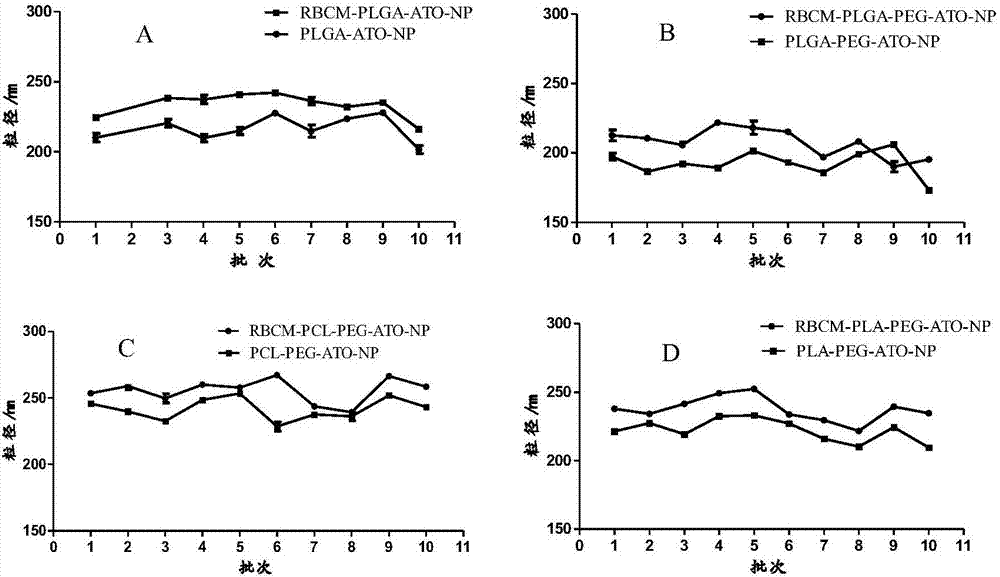
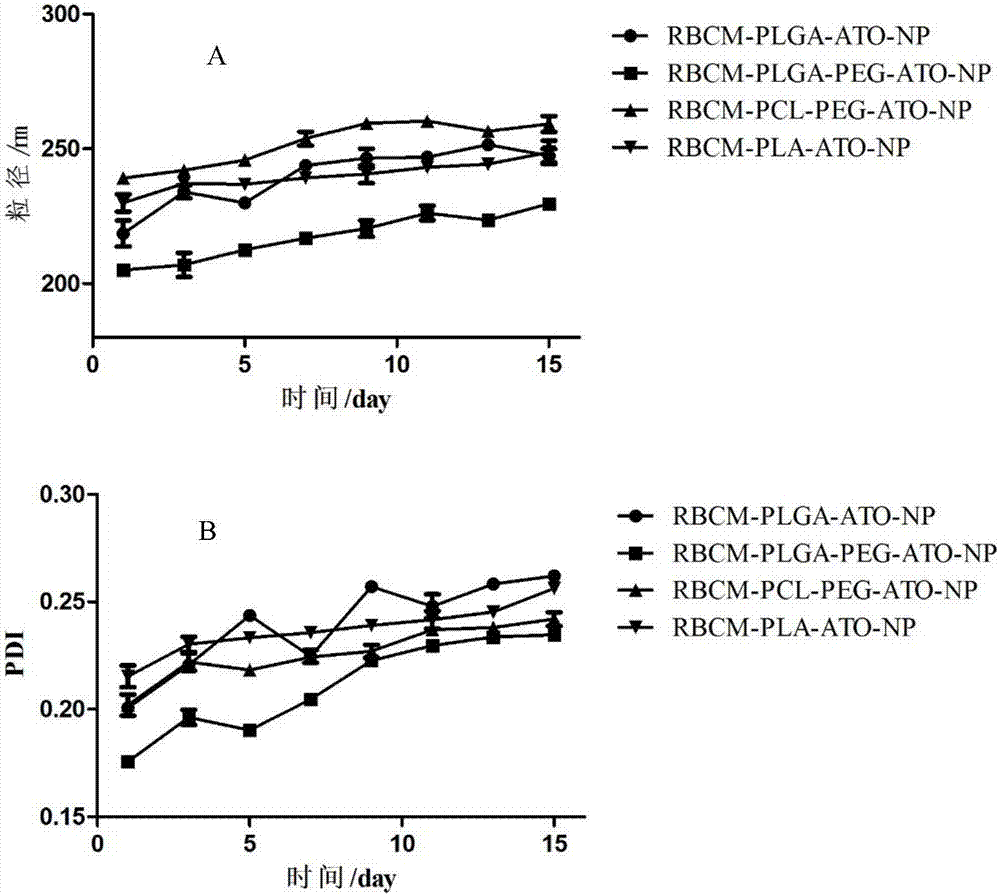
[0041] Preparation by double emulsion method: take 1mL of 10mg / mL polyester material solution, slowly add it into 10mL of the external aqueous phase containing emulsifier under the condition of magnetic stirring, ultrasonication for 2min, and magnetic stirring for 3h to evaporate the organic solvent to form nanoemulsion. The prepared nanoemulsion was put into a dialysis bag with a permeation molecular weight of 30,000, and was dialyzed for 2 hours to remove free drugs to obtain a nanoparticle suspension. Particle size and polydispersity index (PDI) were measured with a Malvern laser particle size analyzer. According to the same preparation method as above, the types and solvents of each material are investigated in the following table 1:
[0042] Table 1
[0043]
[0044] From the screening results of the single factor method in Table 1, it can be seen that the molecular weight of PLGA and PLGA-PE...
Embodiment 3
[0045] Embodiment 3.RBCM-PLGA-ATO-NP preparation
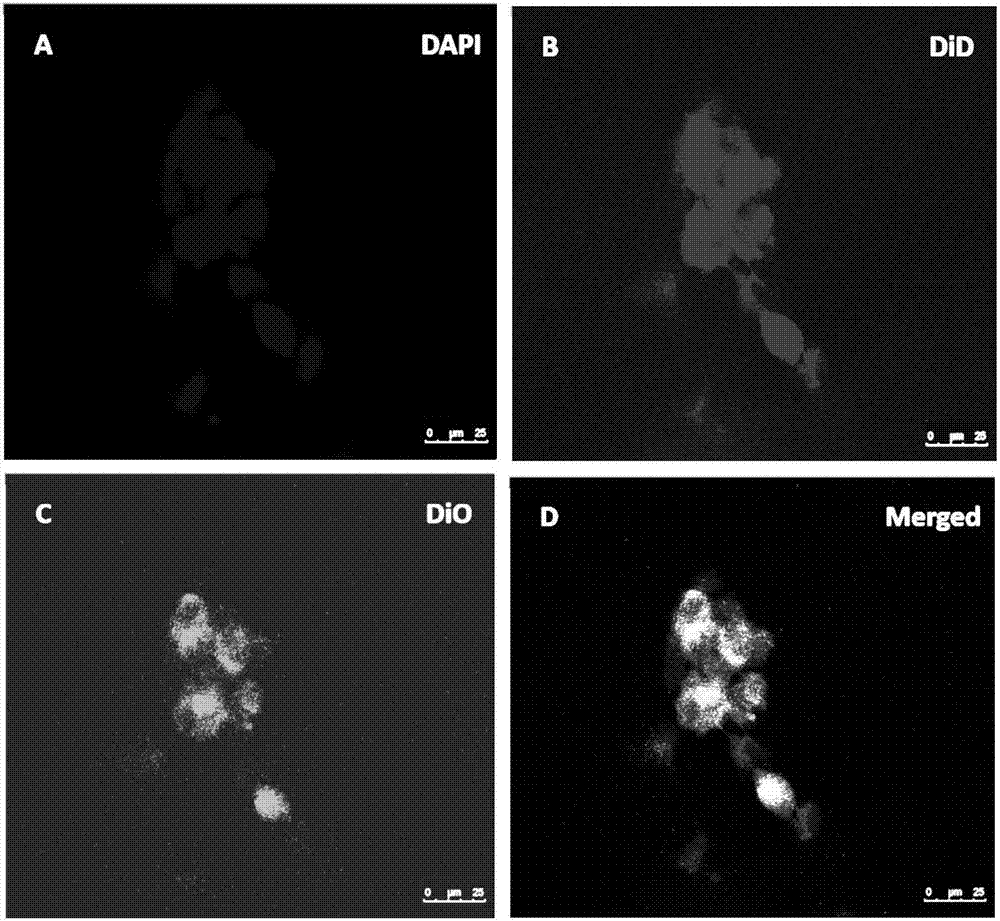
[0046] Add 2mL of arsenic trioxide aqueous solution to the PLGA acetone solution, sonicate until a homogeneous phase is formed, slowly add it into the external aqueous phase containing emulsifier under the condition of magnetic stirring, and evaporate the organic solvent by magnetic stirring to form a nanoemulsion. Rotary evaporate to 1-2mL, add the prepared red blood cell membrane, sonicate, put the nano solution into a dialysis bag (MW 3000), dialyze for 2 hours to remove free drug, and obtain the nanoparticle suspension encapsulated by the red blood cell membrane.
[0047] Described PLGA molecular weight is 3000-95000, and lactic acid: glycolic acid polymerization ratio is 50:50; PLGA consumption is 10mg; The volume of PLGA acetone solution is 1mL;
[0048] The consumption of described arsenic trioxide is 1mg, and the volume of inner aqueous phase is 0.1mL;
[0049] The emulsifier is Paloxamer 188, the dosage is 3 mg, an...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Volume | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com