Tecovirimat dry suspension and preparation method thereof
A technology of Tevirima and dry suspension, which is applied in the field of Tevirima dry suspension and its preparation, can solve the problems of unreported preparation bioavailability and pharmacokinetic parameters, and achieve anti-mousepox virus activity Strong, easy to take, slow sedimentation effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Embodiment 1, investigate the method for improving the monoclinic crystal solubility of Tevir immediately monohydrate
[0034] The use of Tevir immediately has a large effective dose, and its solubility in water is low (the measured value is 1.52 μg / ml), and the addition of acid can not improve the solubility, and the addition of alkali leads to degradation, which poses many challenges to the preparation research of Tevir immediately.
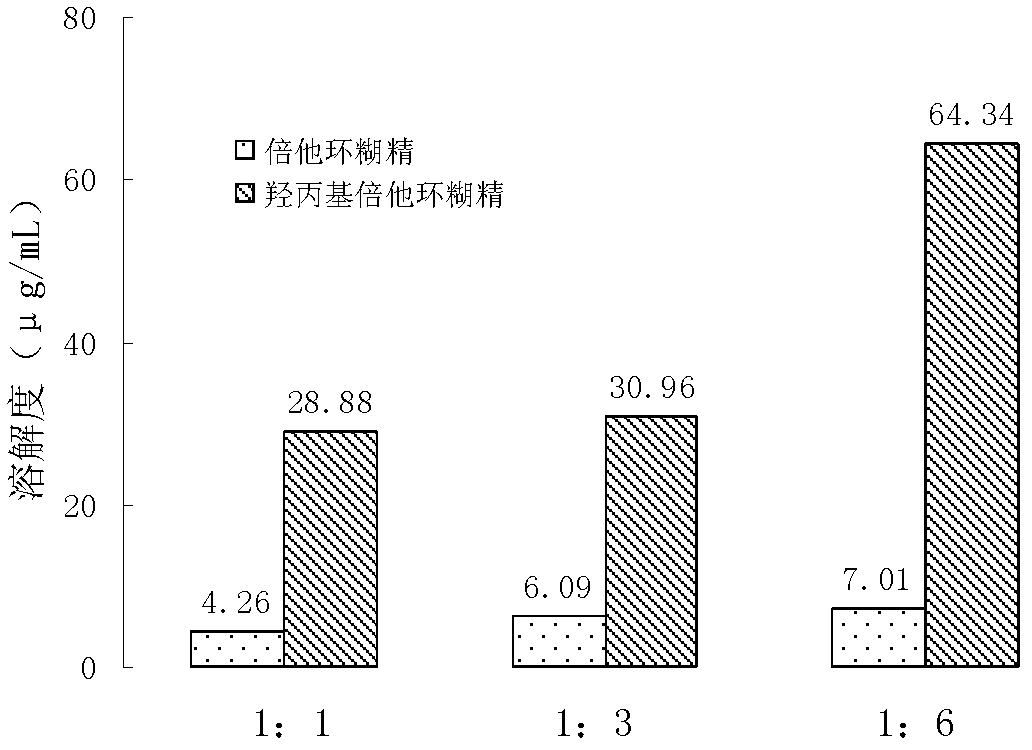
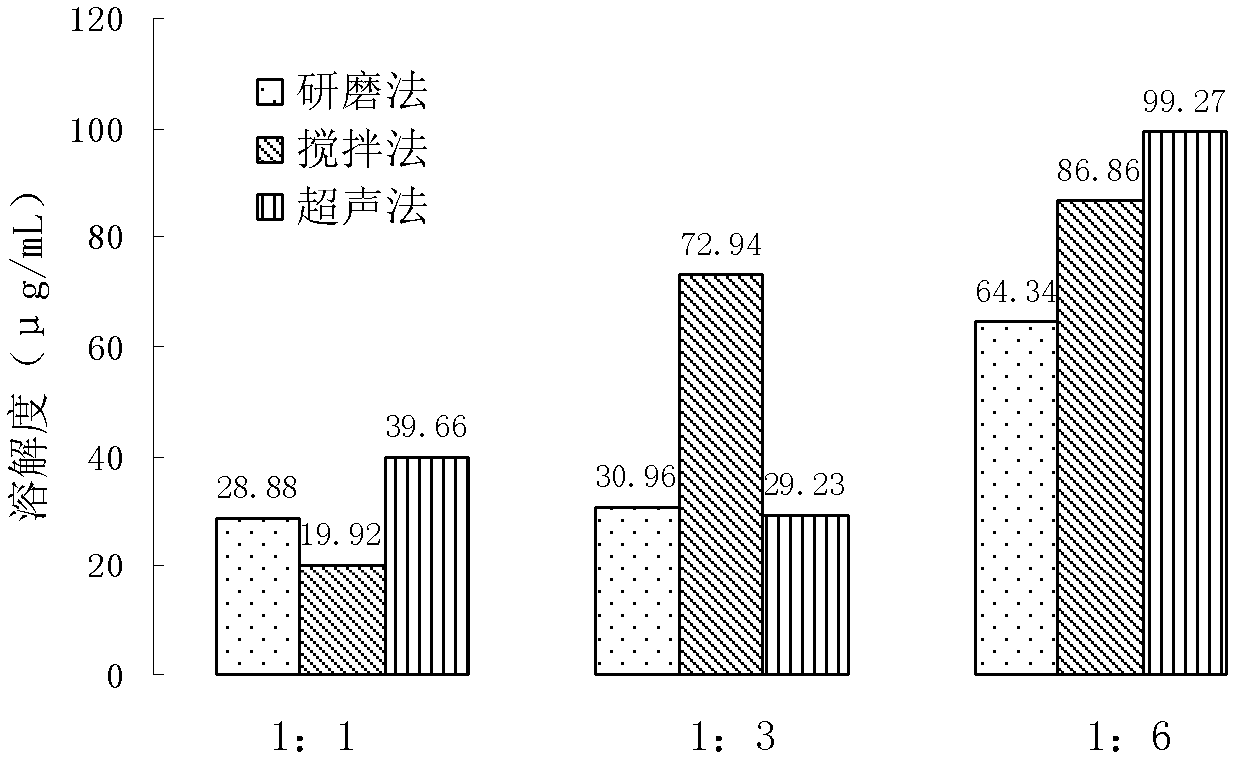
[0035] In order to improve the bioavailability of Tevirima monohydrate crystals, the inventors increased the monoclinic crystals of Tevirima monohydrate by exploring methods such as reducing particle size, adding solubilizers, cyclodextrin inclusions and solid dispersions. Solubility in water.
[0036] 1) Tevir immediately micronized
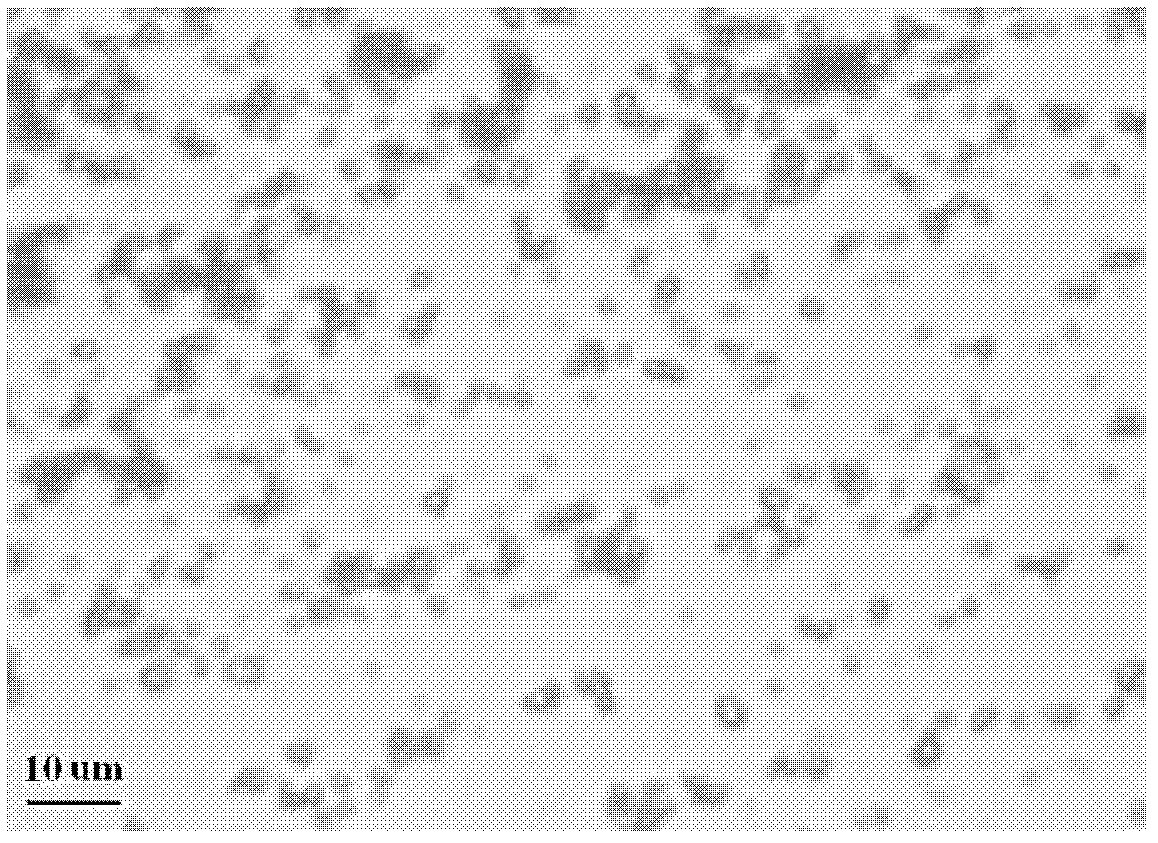
[0037] Micronizing the drug significantly reduces the particle size, increases the surface area of the drug in contact with the solvent, increases solubility, and improves absorption. The monoclinic cr...
Embodiment 2
[0053] Embodiment 2, preliminary screening of Tevir immediate dry suspension prescription
[0054] Investigated the bioavailability of Tevir immediately in different suspending agents and solubilizer formulations, CL-611 (U.S. FMC company) is a product made after special mixing of microcrystalline cellulose and sodium carboxymethyl cellulose, suspending Works well, so try to apply. In addition, in order to improve the wettability of Tevirima, surfactants such as SDS, Tween 80, poloxamer 407 and 188 were added to the trial prescription; while micronized silica gel has the ability of anti-caking and flow aid, which can make the The preparation has good powder properties. Hydroxypropyl beta-cyclodextrin inclusion Tevir can increase the solubility of the drug immediately, and it is also included in the formula screening. Prescription design is shown in Table 2, and each prescription all meets the requirement of two appendix IO of Chinese Pharmacopoeia version in 2010.
[0055] ...
Embodiment 3
[0062] Embodiment 3, the optimization of Tevir immediate dry suspension prescription
[0063] (1) Screening of wetting agents
[0064] Immediately add 500 mg of Tevir to 50 mL of water, and increase the wetting agent according to the gradient of 5 mg, and observe whether Tevir is completely wetted immediately, sinks under the liquid surface, and produces less foam.
[0065] By investigating the wetting effect of several wetting agents such as Tween 80, sodium lauryl sulfate, poloxamer 407 and 188 on Tevir immediately, it was found that when the amount of poloxamer 407 was 20-25mg, there was a significant effect. Good wetting effect.
[0066] (2) Screening of defoamers
[0067] In the suspension state, the wetting agent will generate a lot of bubbles, in order to ensure the wetting of Tevir immediately, only antifoaming agent can be added to reduce the generation of foam. Simethicone oil is odorless, non-toxic, physiologically inert, and has good chemical stability. Since si...
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com