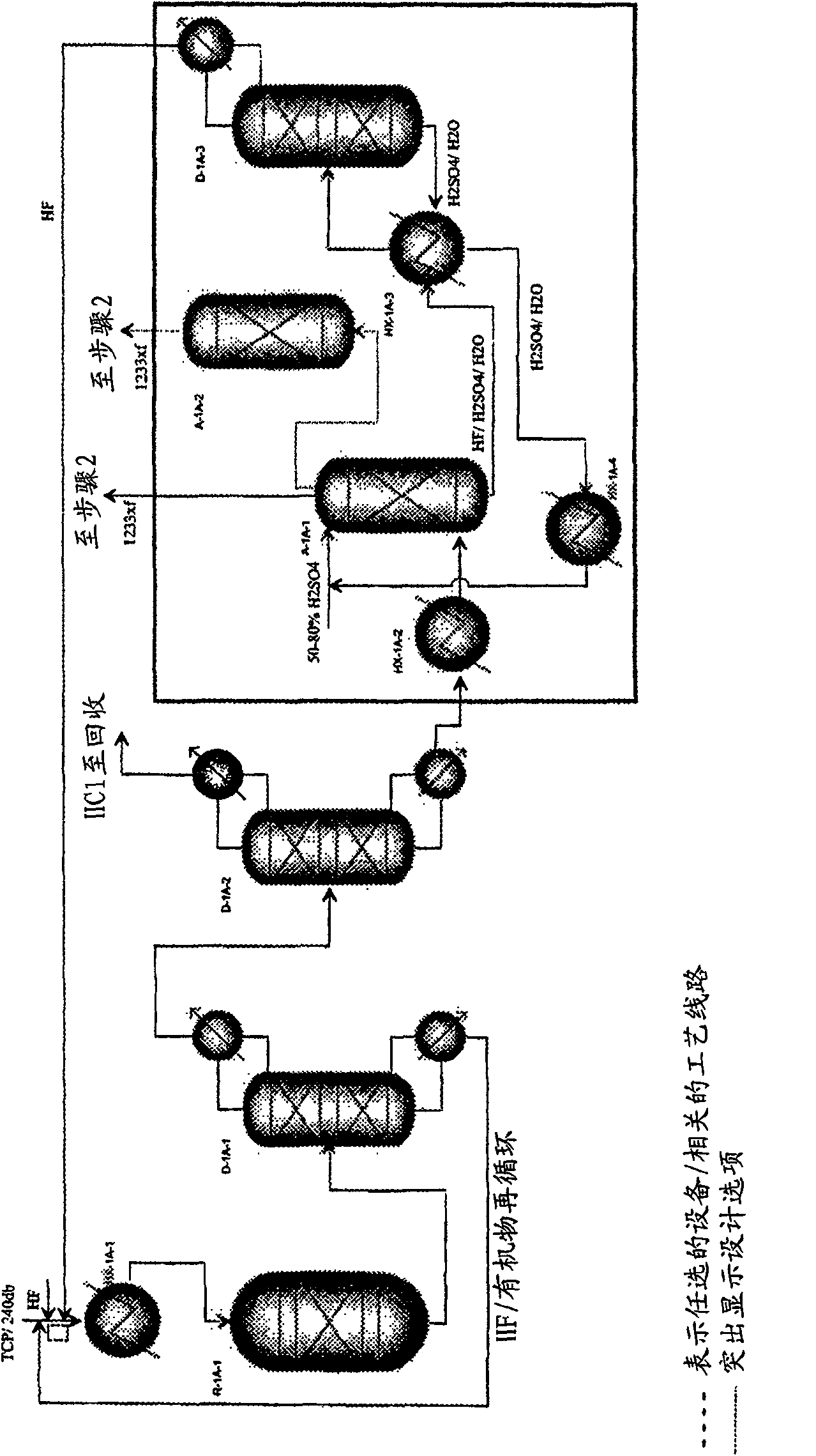
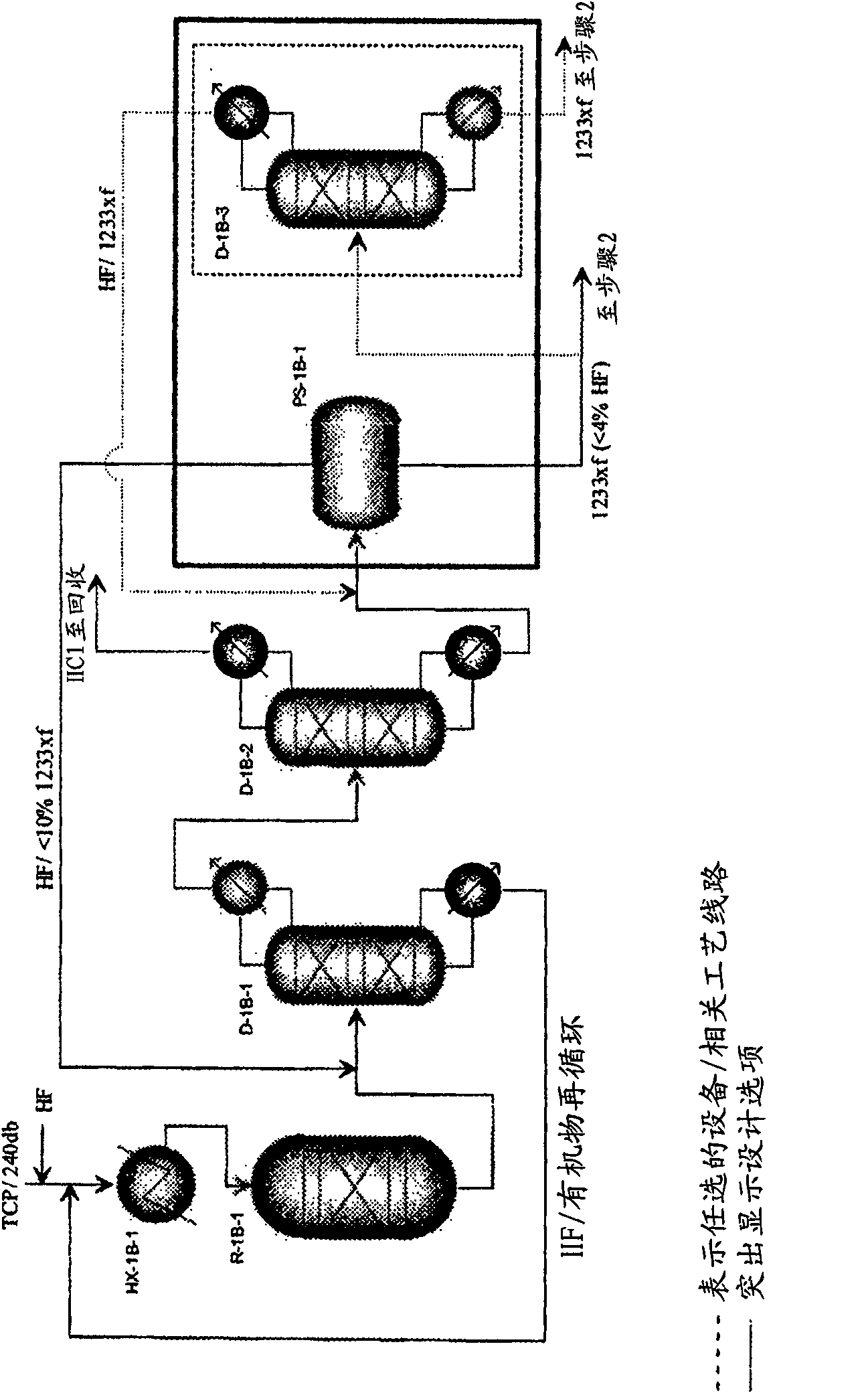
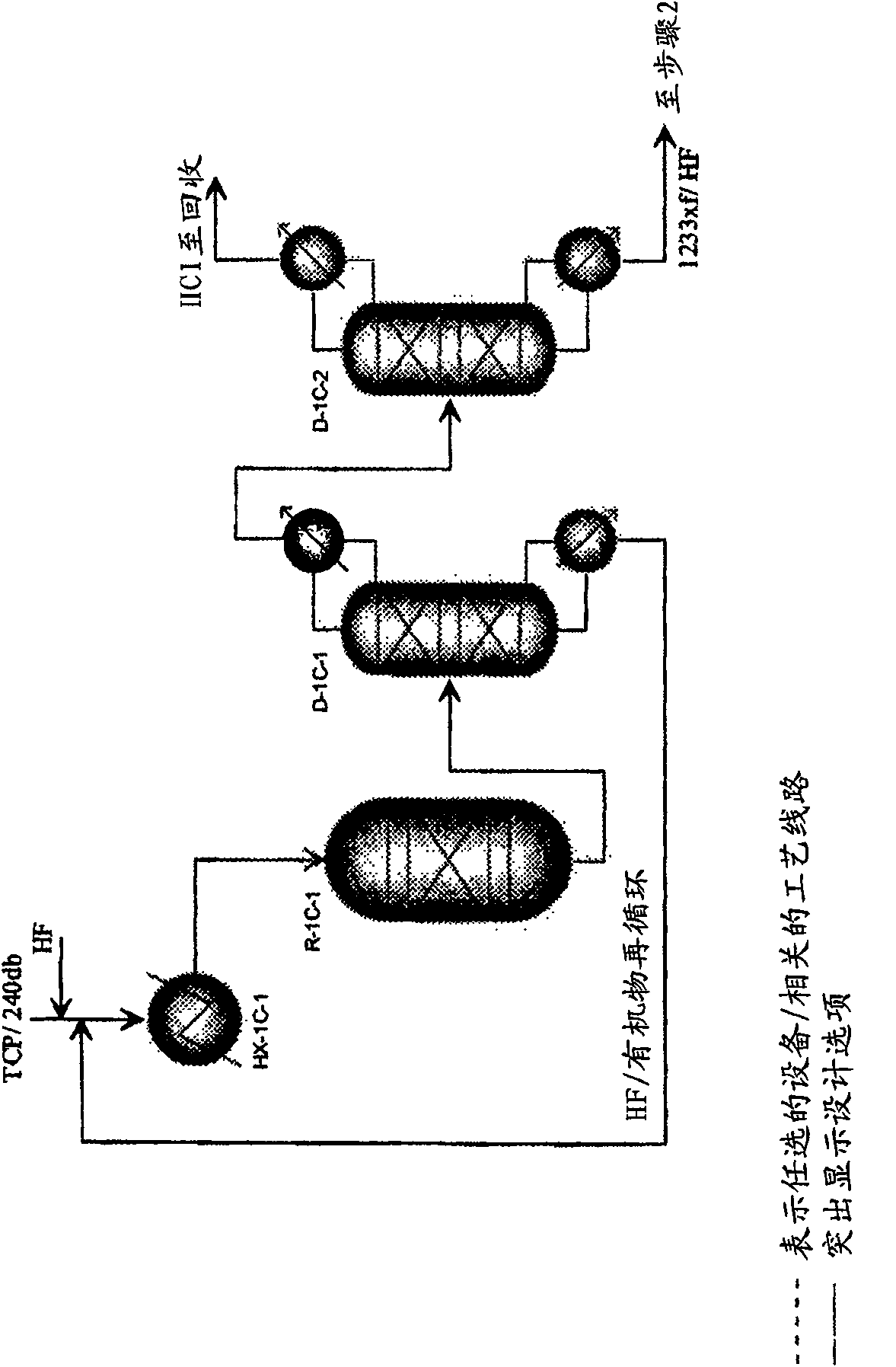
Integrated process to produce 2,3,3,3-tetrafluoropropene
A technology of tetrafluoropropane and tetrafluoropropane, which is applied in the field of preparing fluorinated organic compounds and can solve the problems of low yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0073] This example illustrates the continuous gas phase fluorination reaction of step 1 1,1,2,3-tetrachloropropene (TCP)+3HF→2-chloro-3,3,3-trifluoropropene (HCFO-1233xf)+ 3HCl. The fluorination catalyst used in this experiment was fluorinated Cr 2 o 3 .
[0074] The continuous gas-phase fluorination reaction system used to study this reaction consists of N 2 , HF and organic feed system, feed evaporator, superheater, 4-inch ID Monel reactor, acid scrubber, dryer and product collection system. Charge 9415.2 grams of pretreated Cr in the reactor 2 o 3 catalyst, equal to approximately 6.5 liters of catalyst. After placing the reactor in a constant temperature sand bath, the reactor was heated to a reaction temperature of about 235 °C with N 2 Purge over the catalyst. Reactor pressure was about 3 psig. HF feed was introduced to the reactor (via evaporator and superheater), and N 2 The co-feed form is fed for 15 minutes, at which time N 2 Stream stops. The HF flow rat...
Embodiment 2
[0076] Fluorinated Cr deactivated after 650 hours run time as described in Example 1 2 o 3 The catalyst is regenerated by the following steps.
[0077] When N 2 The reactor was heated to 300°C while the flow rate was 5000cc / min. After the reactor temperature stabilized, synthetic air was introduced. The initial flow rate of air is such that with 0.5% O 2 . Gradually, increase 0.25% O 2 , the air flow increases so that O 2 The concentration is 2.0%. The reactor hot spot rose to 360°C. Then gradually increase the air flow rate, in increments of 0.5-1.0%, so that O 2 The concentration was increased to 5.0%. The reaction heater temperature was carefully adjusted to avoid overheating of the reactor by exceeding 380 °C.
[0078] When flowing 5% O 2 / N 2 At the same time, the reactor temperature was maintained at the hot spot temperature of the catalyst bed at 360-375° C. until the hot spot reached the top of the catalyst bed. Next, without changing the temperature of t...
Embodiment 3
[0081] This example illustrates the continuous gas-phase fluorination reaction of step 1 1,1,1,2,3-pentachloropropane (HCC-240db) + 3HF → 2-chloro-3,3,3-trifluoropropene (HCFO -1233xf)+4HCl. The fluorination catalyst used in this experiment was fluorinated Cr 2 o 3 .
[0082] The same continuous gas phase fluorination reaction system described in Example 1 was used in Example 3. Under the condition that the molar ratio of HF to HCC-240db is 15:1, the contact time is 15 seconds and the reaction temperature is 255°C, the reaction of HCC-240db+3HF→HCFO-1233xf+4HCl is carried out. GC analysis of the reactor effluent showed 100% conversion of HCC-240db and 98.3% selectivity to HCFO-1233xf on a molar basis. See Table 1 for details of Example 3.
[0083] Table 1
[0084] experiment # 71 HCC-240db+3HF→HCFO-1233xf+4HCl Fluorinated Cr 2 o 3 Catalyst, the molar ratio of HF to HCC-240db is 15:1, the contact time is 15 seconds, and the reaction temperature is 255°C.
[0085] ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com