Monoazo compounds, preparation method and uses thereof
A monoazo, compound technology, applied in the field of monoazo compounds, can solve the problems of poor washing fastness, poor washing fastness, difficult to dye deep, etc., and achieves good moisture resistance, good sublimation fastness, and good temperature dependence. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
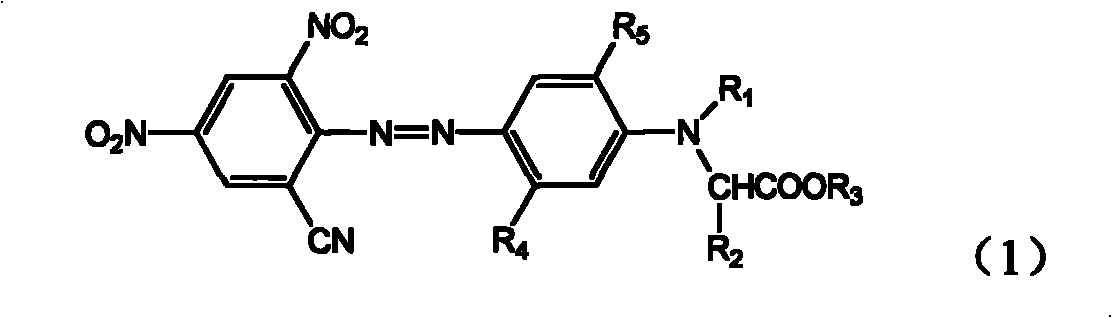
[0037] 2,4-dinitro-6-bromoaniline shown in formula (2)
[0038]
[0039] Carry out diazotization with nitrosyl sulfuric acid in sulfuric acid medium, the diazonium salt that obtains is coupled with the compound shown in formula (3) in acidic medium,
[0040]
[0041] where R 1 , R 2 , R 3 , R 4 and R 5 With the definition of the above-mentioned formula (1), then the obtained coupled compound is reacted with CuCN or NaCN+CuCN in an organic solvent to replace the bromine substituent, and the monoazo compound of the present invention shown in the formula (1) is obtained. compound.
[0042]
[0043] The diazo component (2) is known and readily available commercially, and the coupling component (3) can be prepared by well-known methods.
[0044] The present invention also relates to a mixture containing at least two azo compounds of different structures of the formula (1), which has better application properties. The mixture of the present invention can be prepared ...
Embodiment 2
[0047] Put 66.0ml of sulfuric acid into the flask, add 52.4g of 2,4-dinitro-6-bromoaniline at controlled room temperature, continue to stir for 30 minutes, cool with ice bath to reach 20-30°C, at this temperature 68.0g 42% nitrosyl sulfuric acid was added dropwise to the reaction mixture, and stirring was continued at this temperature for 4 hours. After completing the reaction, the mixture was dropped into a mixture of 30.0ml sulfuric acid, 900.0g ice water and 56.0g of the compound of the following formula within 30 minutes at 0-5°C,
[0048]
[0049] Stirring was continued for 2 hrs, then 1000 ml of water was added, and the temperature was slowly raised to 60° C. over 1 hour and kept for 1 hr. The resulting suspension was suction filtered, washed with water and dried.
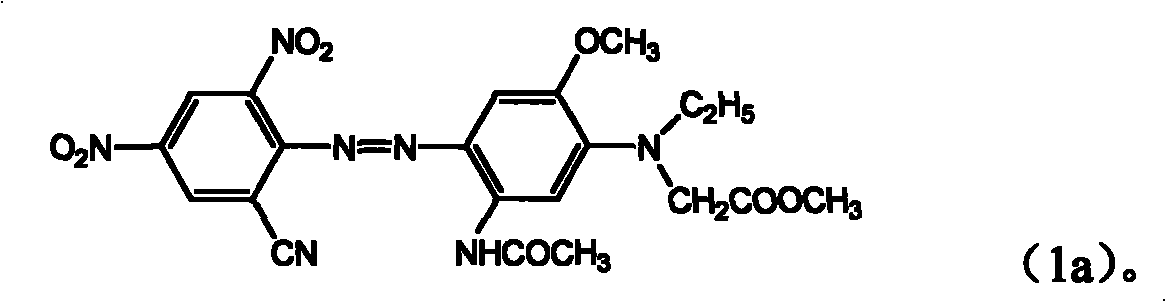
[0050] A dye of the formula is obtained,
[0051]
[0052] Stir and mix 55.0g dry product of the above formula (4), 9.8g cuprous cyanide and 300g DMF, slowly raise the temperature to 60°C in 1 hour an...
Embodiment 3
[0058]Put 66.0ml of sulfuric acid into the flask, add 52.4g of 2,4-dinitro-6-bromoaniline at controlled room temperature, continue to stir for 30 minutes, cool with ice bath to reach 20-30°C, at this temperature 68.0g 42% nitrosyl sulfuric acid was added dropwise to the reaction mixture, and stirring was continued at this temperature for 4 hours. After completing the reaction, the mixture was dropped into a mixture of 30.0ml sulfuric acid, 900.0g ice water and 50.5g of the compound of the following formula within 30 minutes at 0-5°C,
[0059]
[0060] Stirring was continued for 2 hrs, then 1000 ml of water was added, and the temperature was slowly raised to 60° C. over 1 hour and kept for 1 hr. The resulting suspension was suction filtered, washed with water and dried.
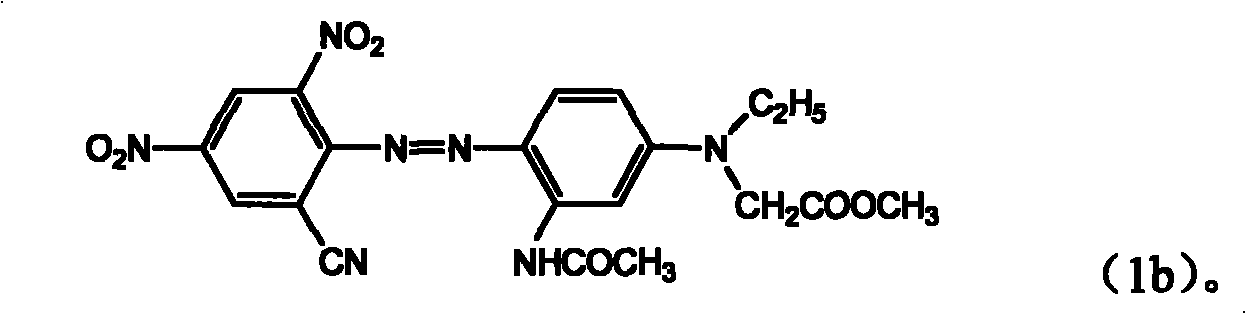
[0061] A dye of the formula is obtained,
[0062]
[0063] Stir and mix 52.6g of the dry product of the above formula (4b), 9.8g of cuprous cyanide and 300g of DMF, slowly heat up to 60°C in 1 hour and...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com