Method for recovering organic chloride from rectification residues of methane chloride or/and tetrachloroethylene
A technology of methane chloride and tetrachloroethylene, applied in the field of chemistry, can solve the problems of waste of organic chloride resources, complicated processes, and many equipment, and achieve the effects of improving income, simple process and less equipment investment.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
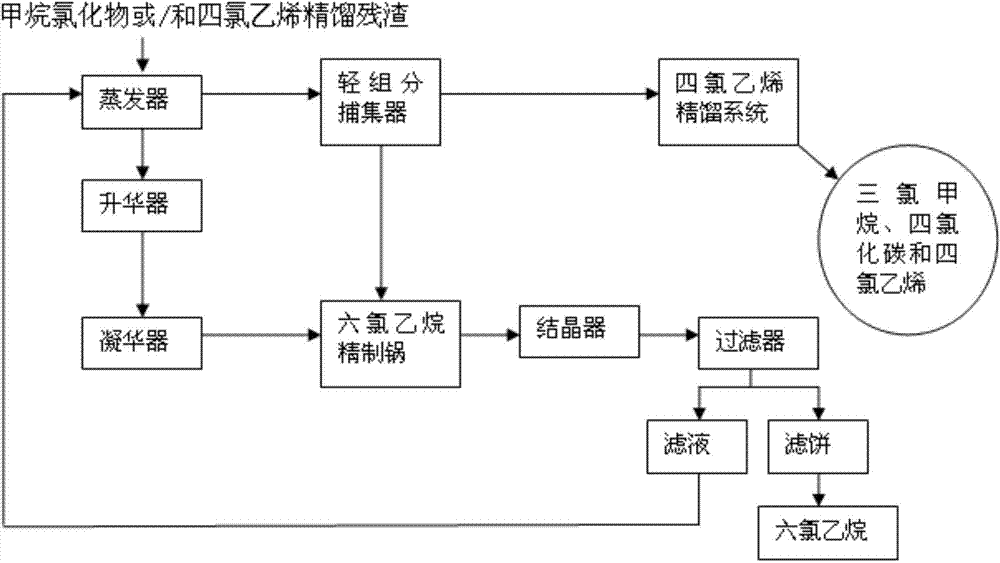
[0018] The method for reclaiming organic chlorides from the rectification residue of methane chlorides, its process route is as follows figure 1 As shown, it specifically includes the following steps:
[0019] (1) Put the rectification residue of methane chloride into the evaporator, turn on the vacuum pump, keep it warm for 30 minutes under the conditions of vacuum degree of 0.05MP and temperature of 145°C, condense the evaporated volatile components into liquid and enter the trap In the collector, the light component mixed liquid is obtained, and the material in the evaporator is transferred to the sublimator while it is hot;
[0020] (2) Pump 60% of the total volume of the light component mixed liquid obtained in the trap in step (1) into the tetrachlorethylene rectification system to separate chloroform, carbon tetrachloride and tetrachloroethylene; The obtained chloroform and carbon tetrachloride are used as raw materials for the production of tetrachlorethylene, and tet...
Embodiment 2
[0027] The method for reclaiming organic chlorides from the rectification residue of tetrachlorethylene, its process route is as follows figure 1 As shown, it specifically includes the following steps:
[0028] (1) Put the rectification residue of tetrachlorethylene into the evaporator, turn on the vacuum pump, keep it warm at a vacuum degree of 0.01MP and a temperature of 140°C until no liquid evaporates, and condense the evaporated volatile components into liquid After entering the trap, the light component mixed liquid is obtained, and the material in the evaporator is transferred to the sublimator while it is hot;
[0029] (2) Pump 50% of the total volume of the light component mixed liquid obtained in the trap in step (1) into the tetrachlorethylene rectification system to separate chloroform, carbon tetrachloride and tetrachloroethylene; The obtained chloroform and carbon tetrachloride are used as raw materials for the production of tetrachlorethylene, and tetrachloreth...
Embodiment 3
[0036] The method for reclaiming organic chloride after mixing the rectification residue of methane chloride and tetrachloroethylene, its process route is as follows figure 1 As shown, it specifically includes the following steps:
[0037] (1) Mix the rectification residue of methane chloride and tetrachlorethylene according to the ratio of 1:1, then pass it into the evaporator, turn on the vacuum pump, and keep warm at a vacuum degree of 0.1MP and a temperature of 150°C until no liquid evaporates So far, the evaporated volatile component is condensed into a liquid and then enters the trap to obtain a light component mixed liquid, and the material in the evaporator is transferred to the sublimator while it is hot;
[0038] (2) Pump 70% of the total volume of the light component mixed liquid obtained in the trap in step (1) into the tetrachlorethylene rectification system to separate chloroform, carbon tetrachloride and tetrachloroethylene; The obtained chloroform and carbon t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com