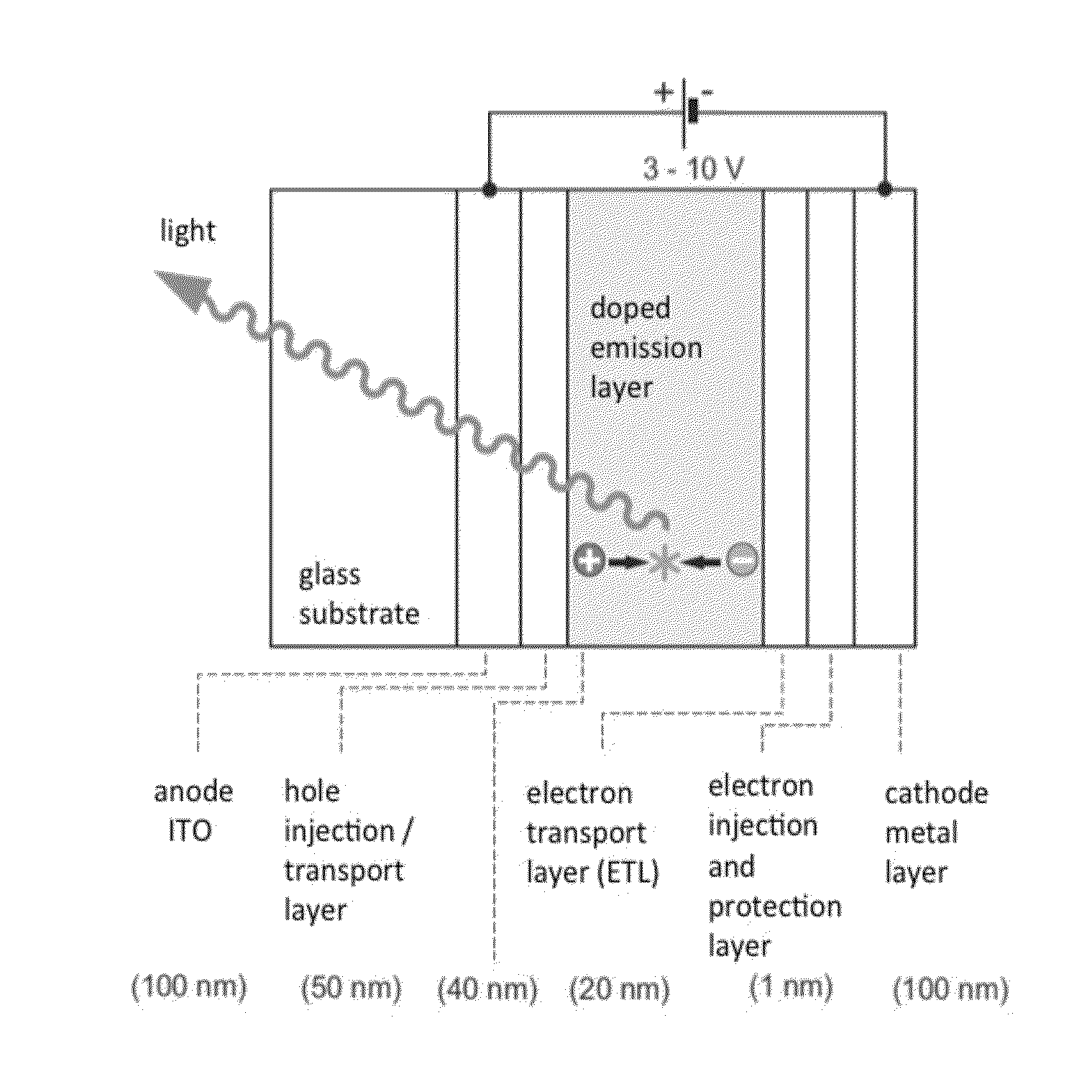
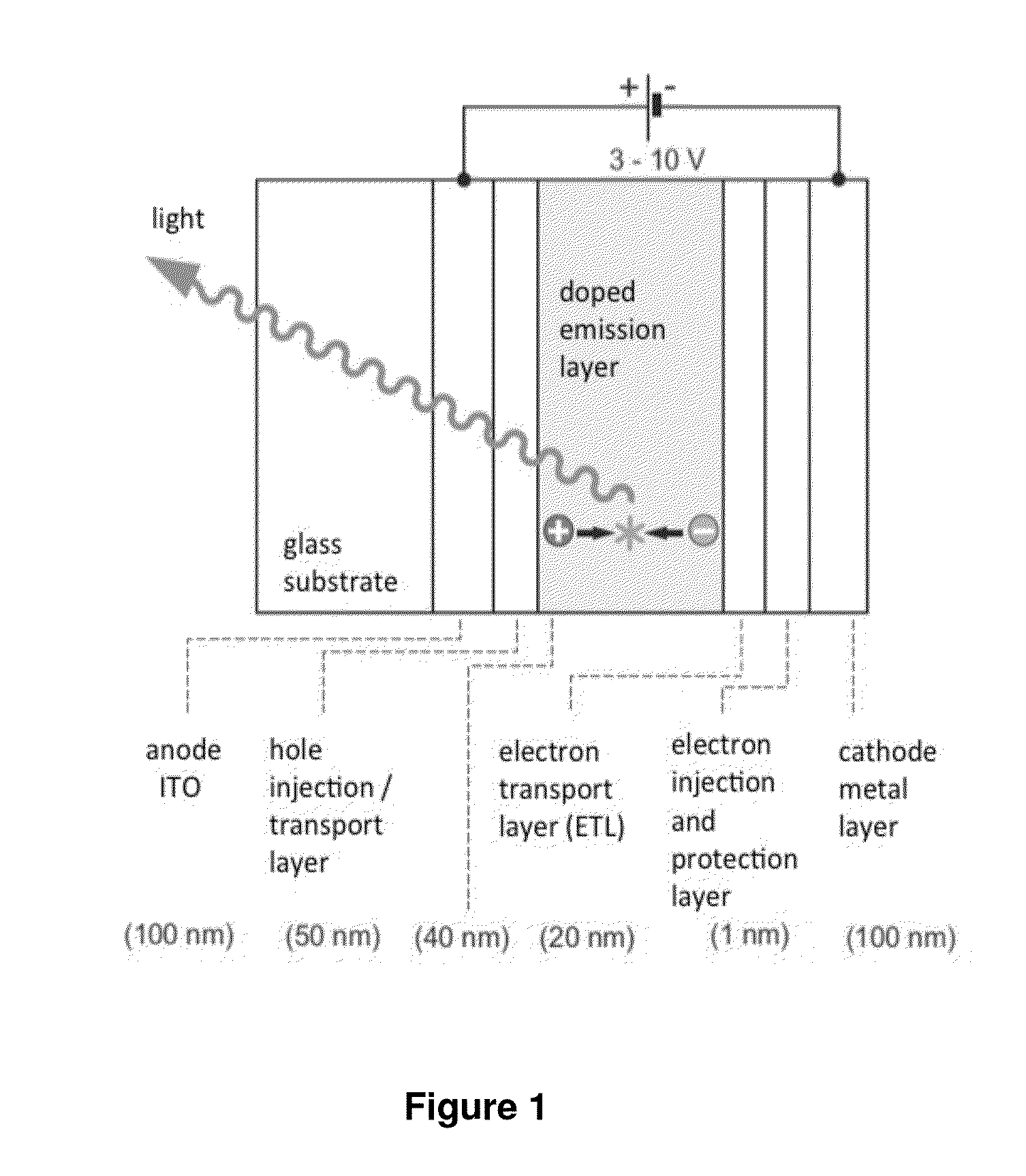
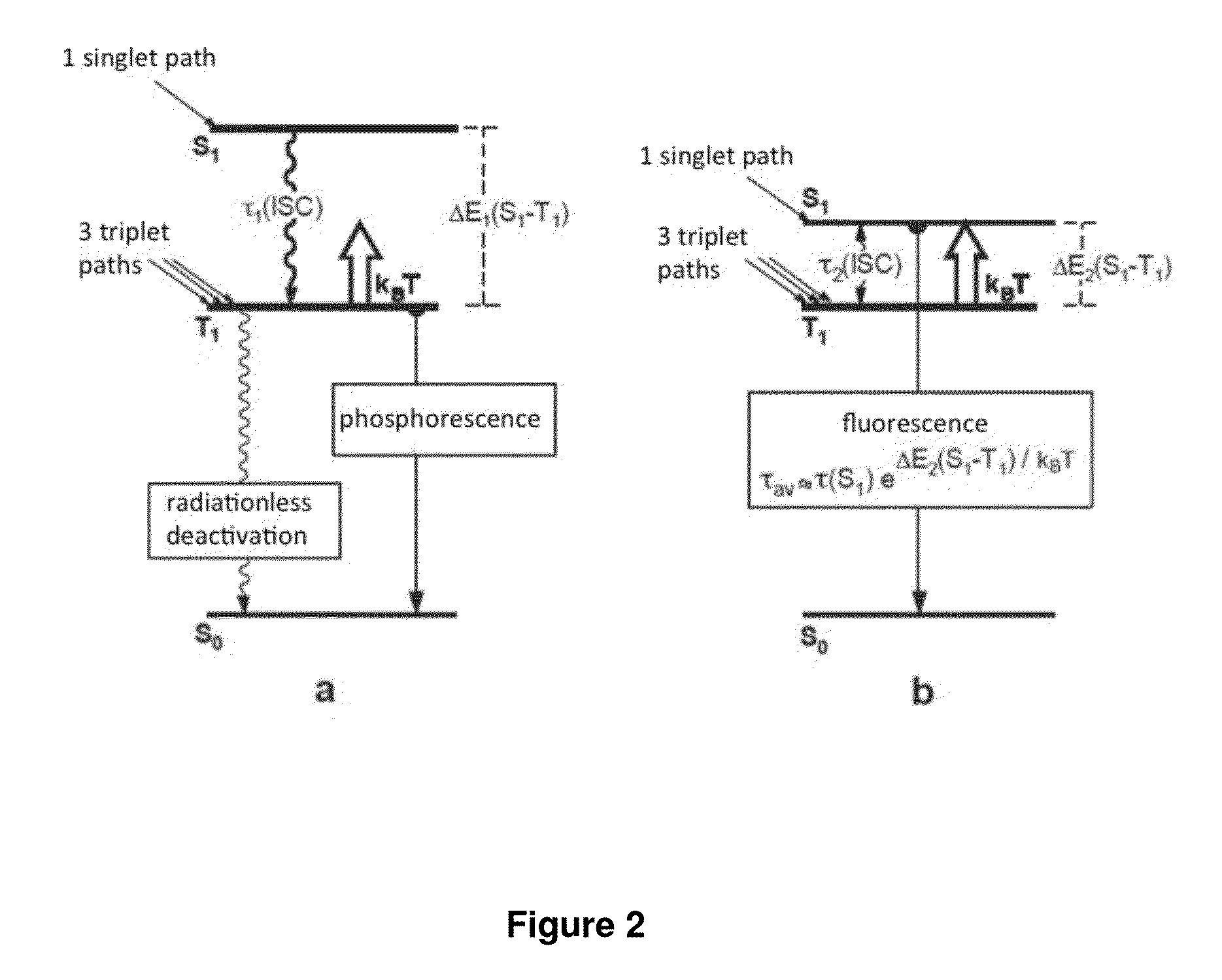
Copper (i) complexes for optoelectronic devices
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
1,10-Phenanthroline Ligands that Increase Solubility in Nonpolar Solvents
2,9-dimethyl-4,7-di(n-butyl)-1,10-phenanthroline, Phen2
[0112]4,7-dichloro-2,9-dimethyl-1,10-phenanthroline, Phen1, is synthesized according to the literature (M. Schmittel, H. Ammon Eur. J. Org. Chem. 1998, 785.). For the substitution with a n-butyl group, an equimolar amount of n-HexMgBr and CuBr is added. The purification of Phen2 is done using column chromatography over silica gel.
example 2
5,8-di(n-butyl)-1,2,3,4,9,10,11,12-octahydrodibenzo[b,j]-[1,10]phenanthroline, Phen4
[0113]The phenanthroline-dichloride Phen3 is synthesized according to (M. Schmittel, H Ammon Eur. J. Org. Chem. 1998, 785.). The synthesis of Phen4 is performed analogously to Phen2.
example 3
2,4,7,9-tetra(n-heptyl)-1,10-phenanthroline, Phen7
[0114]2,4,7,9-tetra-methyl-1,10-phenanthroline, Phen5, is synthesized according to (G. Butt, R. D. Topsom, J. Heterocyclic Chem. 1981, 18, 641). 2,4,7,9-Tetrabromomethylen-1,10-phenanthroline, Phen6, is synthesized via side chain bromation using NBS and isolated by column chromatography (SiO2). The reaction with n-HexLi leads to Phen7.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com