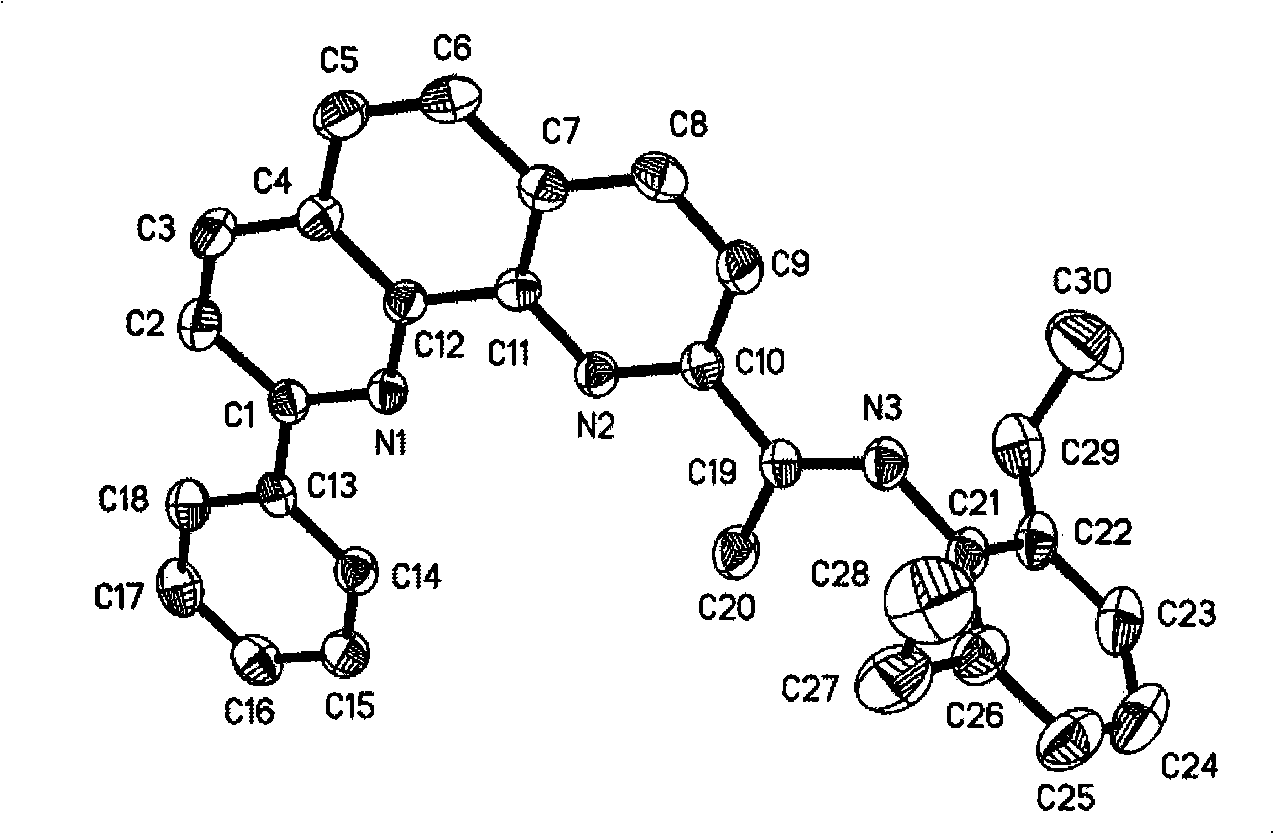
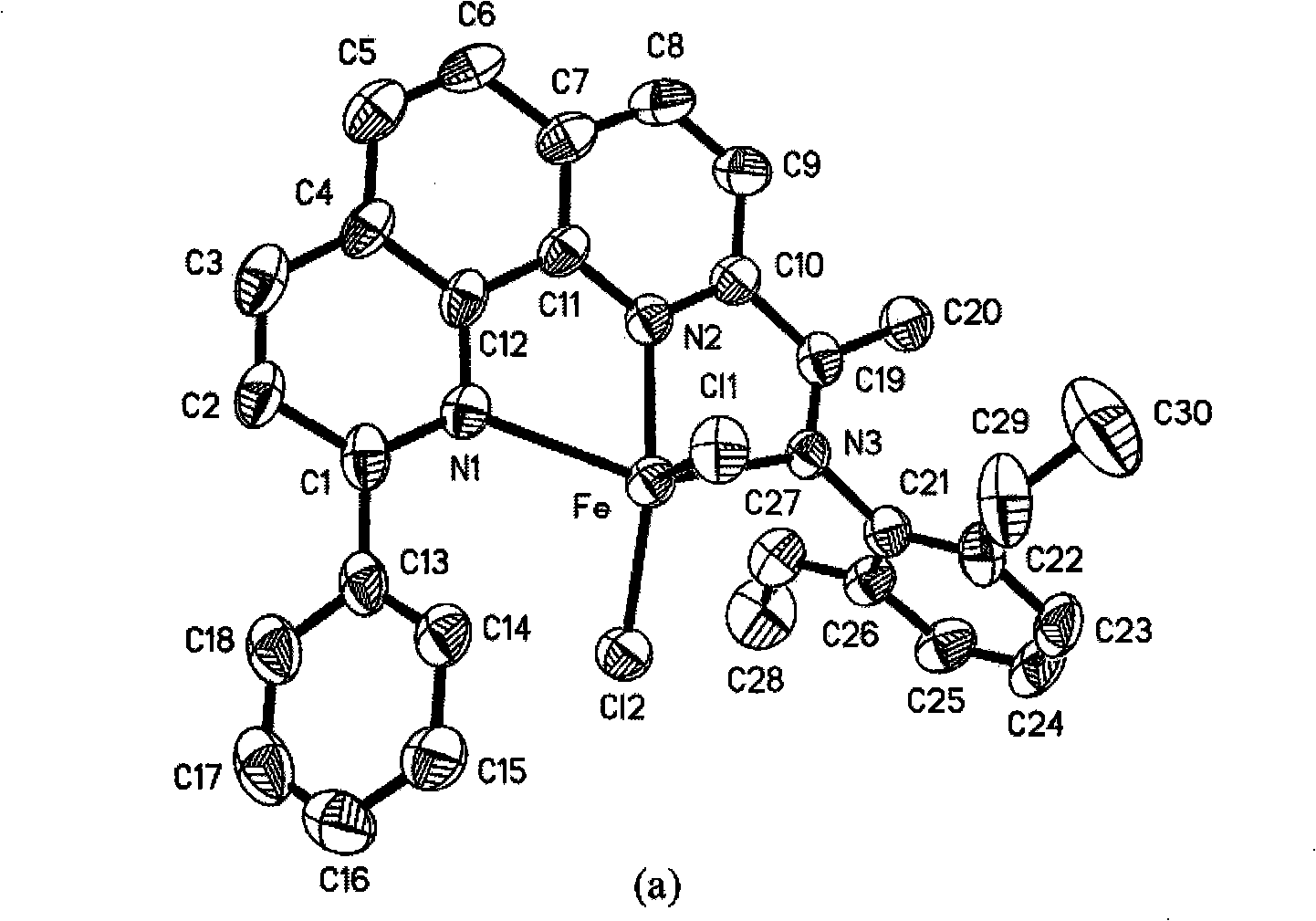
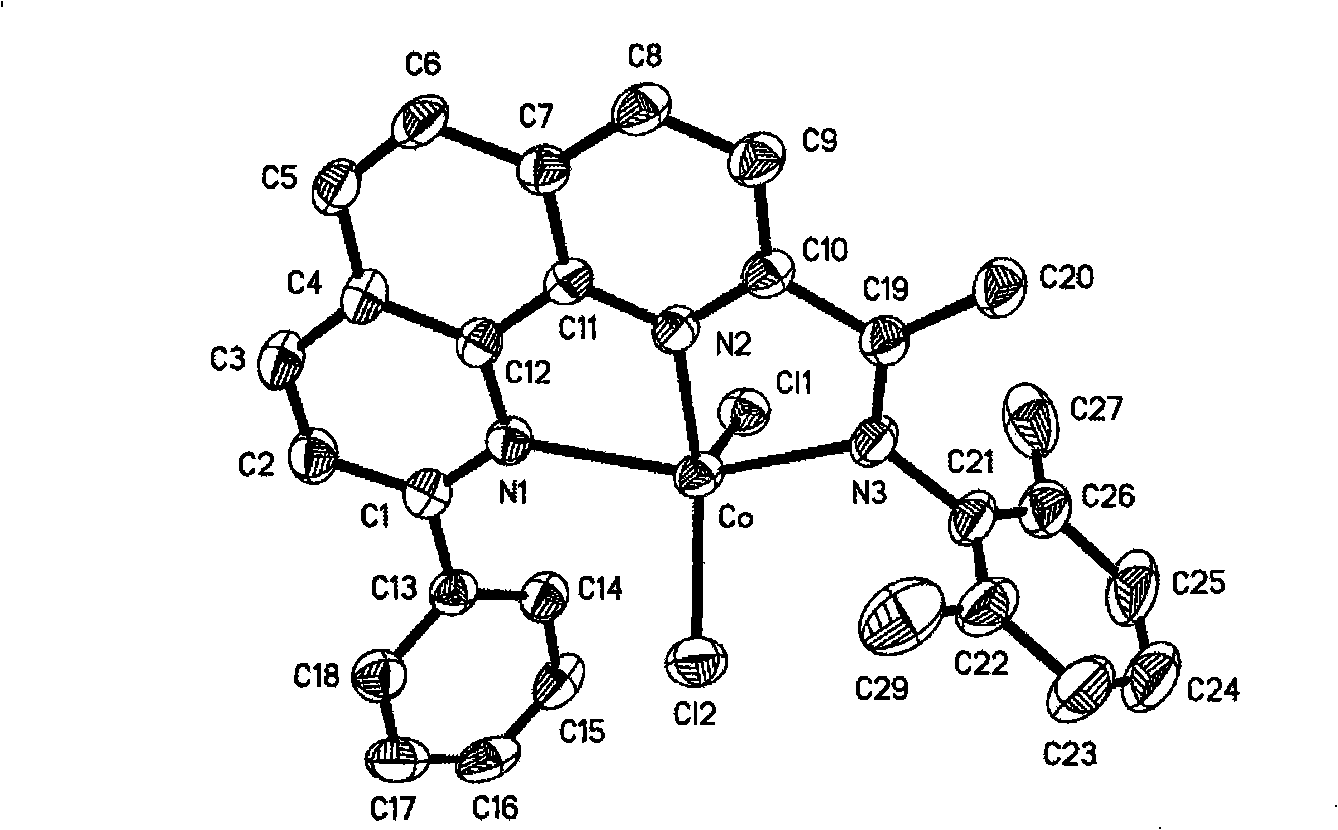
2-imino-9-phenyl-1,10-phenanthroline transient metal complex, and preparation and use thereof
A transition metal and imine-based technology, applied in the field of 2-imino-9-phenyl-1,10-phenanthroline transition metal complexes and their preparation and application, can solve the problem of harsh operating conditions and blockage It can achieve high 1-butene selectivity, excellent catalytic activity, and wide industrial application prospects.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0061] Concrete preparation process is as follows:
[0062] 1. General method of ligand synthesis
[0063] 1.2-Acetyl-9-phenyl-1,10-phenanthroline and alkyl-substituted aniline were refluxed in toluene with p-toluenesulfonic acid as a catalyst for 1 day, after the reaction solution was concentrated, it was perbasic alumina column, and petroleum After washing with ether / ethyl acetate (20:1), the second fraction was divided into the product, and the solvent was removed to obtain a yellow solid, which was the corresponding ligand.
[0064] 2.2-Benzoyl-9-phenyl-1,10-phenanthroline and alkyl-substituted aniline use p-toluenesulfonic acid as a catalyst, use ethyl orthosilicate as solvent and dehydrating agent, at 140-150°C Heat the reaction for 1.5 days, remove tetraethyl orthosilicate under reduced pressure, then rinse the perbasic alumina column with petroleum ether / ethyl acetate (6:1), the second stream is divided into products, and the solvent is removed to obtain a yellow soli...
Embodiment 1
[0072] 1. Preparation of 2-acetyl-9-phenyl-1,10-phenanthroline:
[0073] 1) 2-acetyl-1,10-phenanthroline (2.823g, 10.0mmol), ethylene glycol (3.254g, 52.4mmol) and p-toluenesulfonic acid (206mg) were refluxed in toluene (100mL) for 18 hours until No water was generated in the oil-water separator, and after removing the solvent toluene, the residue was purified by an alkaline alumina column, rinsed with petroleum ether / ethyl acetate (1:2), and the second stream was divided into products, and a light yellow solid was obtained after concentration 1.691 g, 50% yield. The melting point is 119-121°C.
[0074] FT-IR (KBr disc): 3054, 2993, 2980, 2938, 2900, 1619, 1589, 1552, 1505, 1490, 1476, 1445, 1391, 1376, 1256, 1232, 1204, 1147, 1129, 1106, 1029, 1009, 953, 855, 789, 752, 675cm-1.
[0075] 1H NMR (400MHz, CDCl3): δ=9.24(d, J=4.4Hz, 1H), 8.25(d, J=8.4Hz, 1H), 8.22(d, J=8.4Hz, 1H), 7.93(d, J=8.4Hz, 1H), 7.77(s, 2H), 7.61(dd, J=4.4Hz, 1H), 4.18(t, J=6.4Hz, 2H), 4.01(t, J=6.4Hz...
Embodiment 2
[0093] 1. The preparation of complex 1 is the same as in Example 1.
[0094] 2. Ethylene oligomerization at normal pressure: Dry a 250ml three-necked round bottom flask equipped with a magnetic stirrer at 130°C for 6hrs continuously, vacuumize while hot and use N 2 Air replacement 3 times. 2.6 mg (5 μmol) of complex 1 were added and then evacuated and replaced with ethylene 3 times. Inject 30 ml of toluene with a syringe, and then add 1.3 ml of modified methylaluminoxane (MMAO) (1.93 mol / l heptane solution) to make Al / Fe=500. At 20°C, the ethylene pressure was maintained at 1 atm, and the reaction was vigorously stirred for 30 min. After the reaction, a small amount of the mixture was taken out with a syringe and neutralized with 5% dilute hydrochloric acid for GC analysis: the oligomerization activity was 4.78×10 5 g·mol -1 (Fe)·h -1 , the resulting oligomer is only 1-butene. The remaining mixture was neutralized with 5% hydrochloric acid in ethanol, and no polymer was ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com