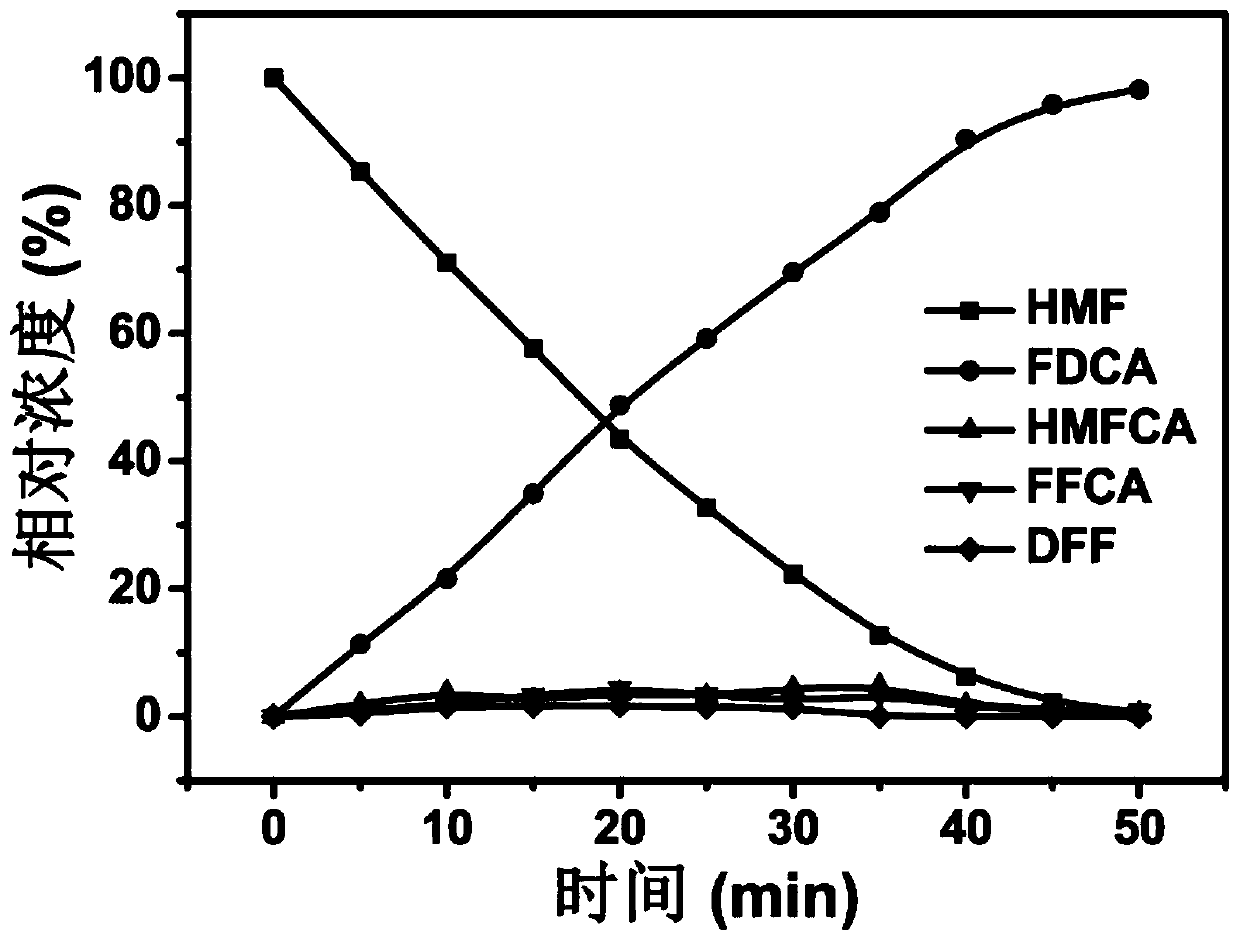
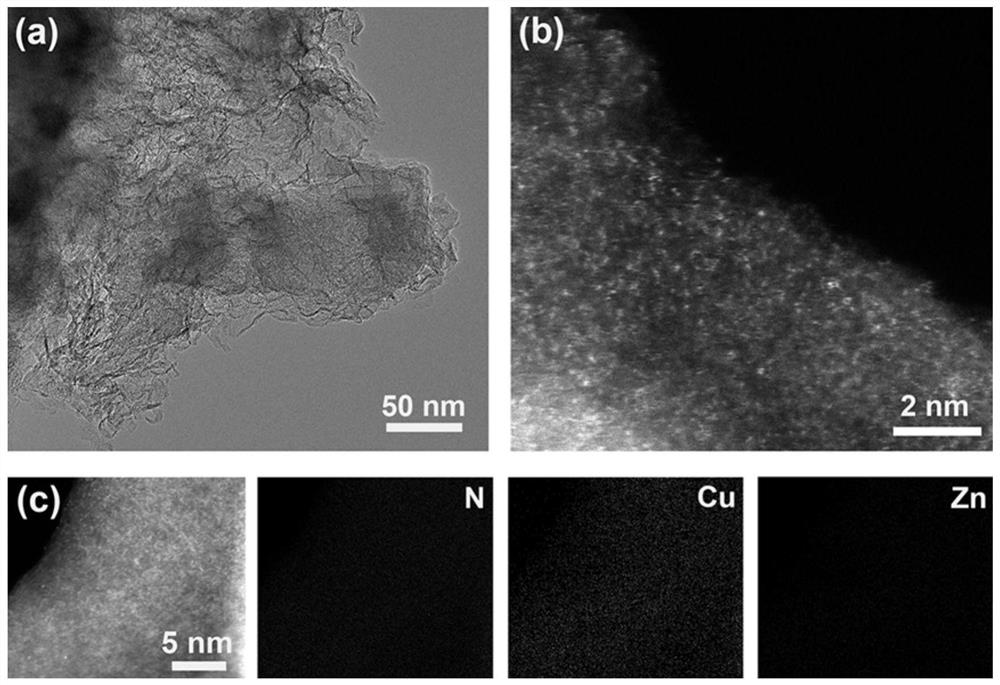
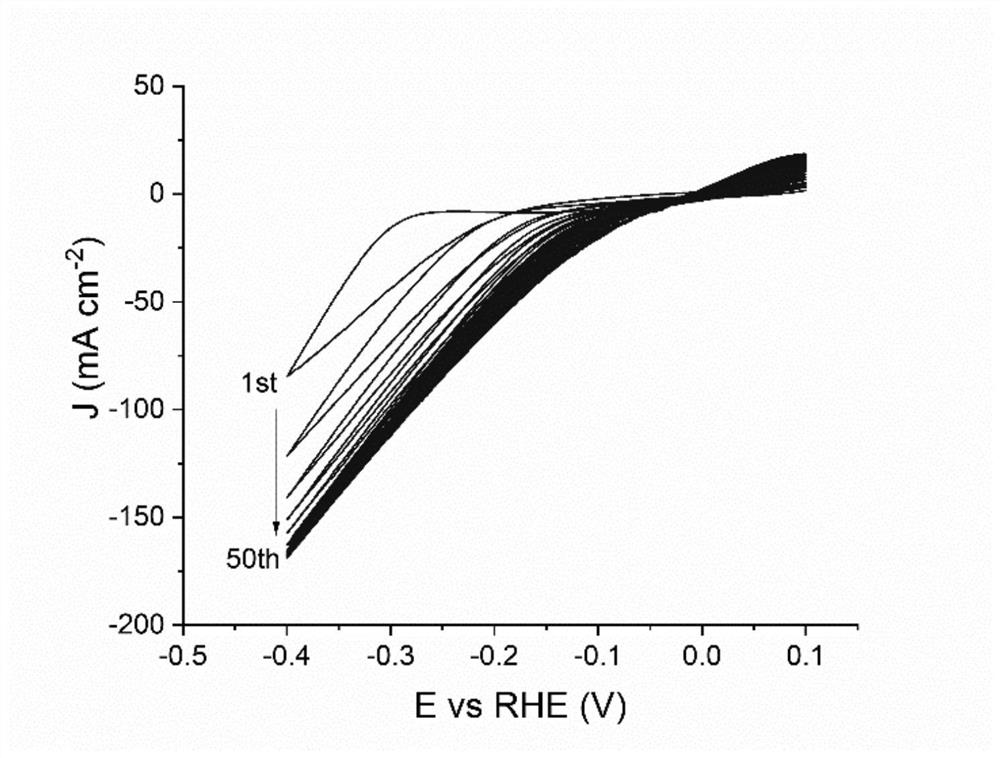
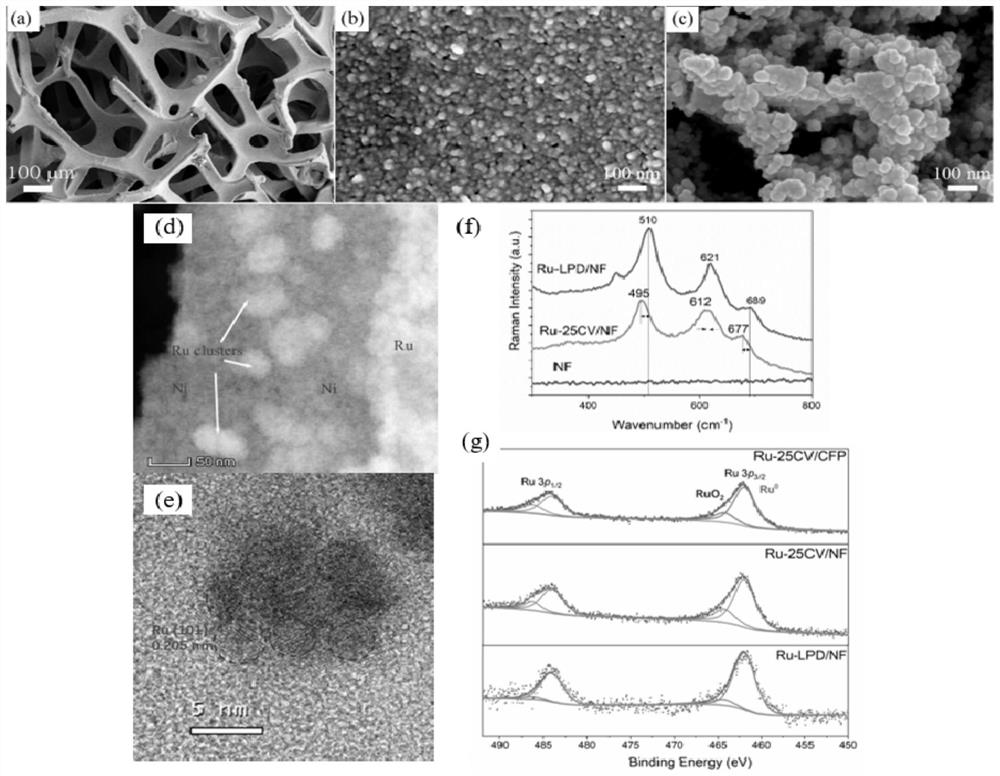
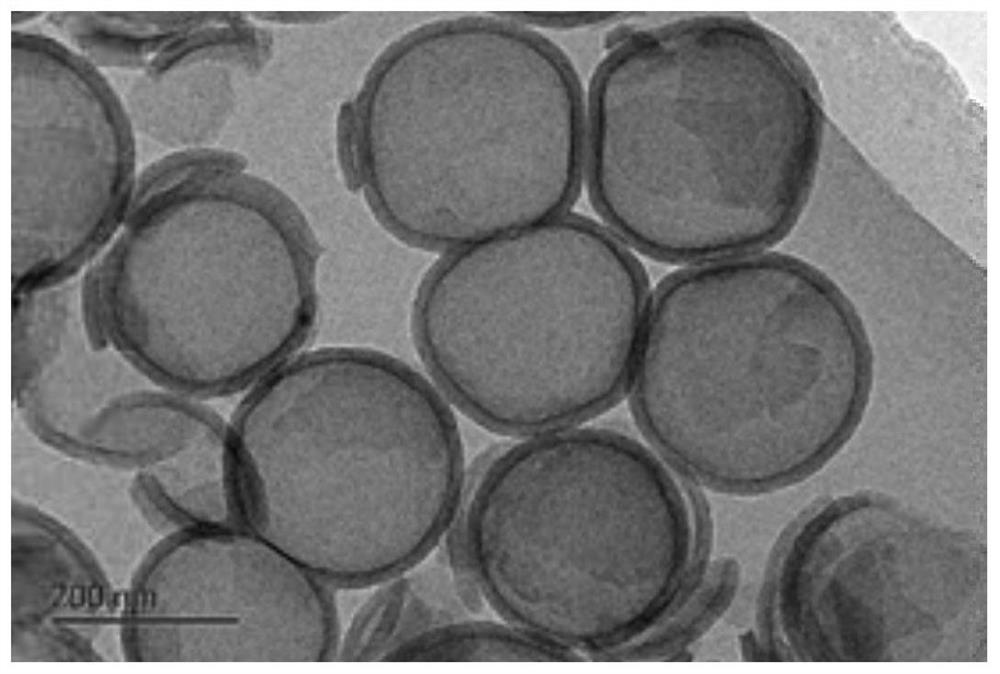
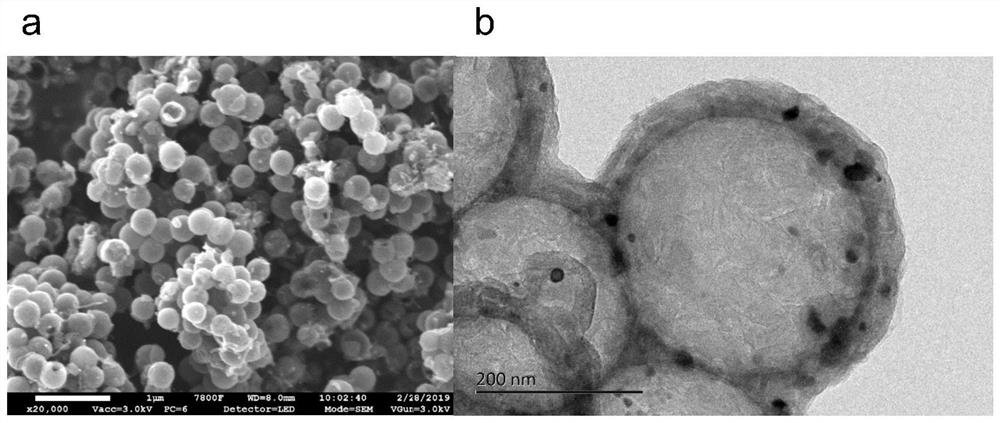
Patents
Literature
266 results about "Faraday efficiency" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Faraday efficiency (also called faradaic efficiency, faradaic yield, coulombic efficiency or current efficiency) describes the efficiency with which charge (electrons) is transferred in a system facilitating an electrochemical reaction. The word "faraday" in this term has two interrelated aspects. First, the historic unit for charge is the faraday, but has since been replaced by the coulomb. Secondly, the related Faraday's constant correlates charge with moles of matter and electrons (amount of substance). This phenomenon was originally understood through Michael Faraday's work and expressed in his laws of electrolysis.
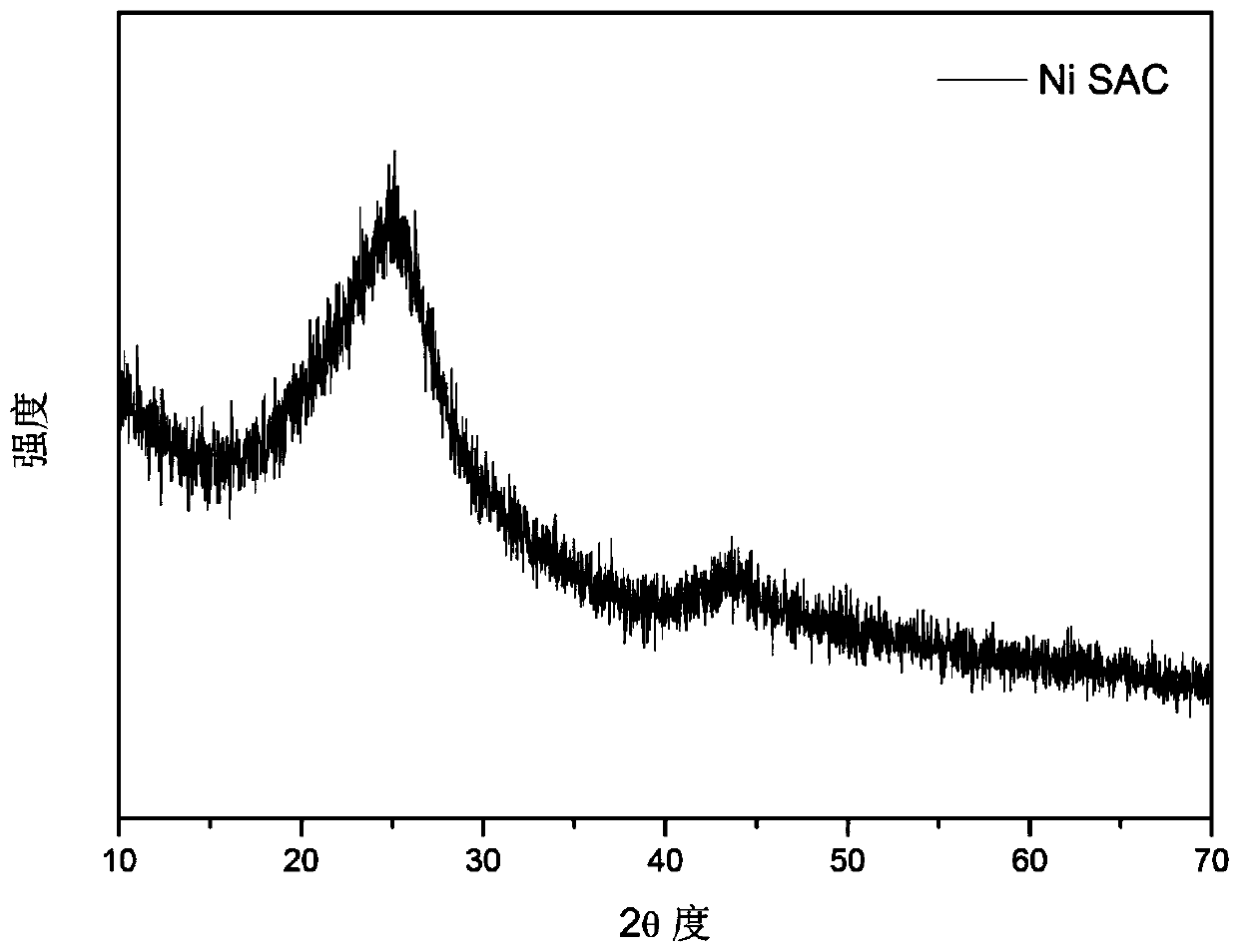
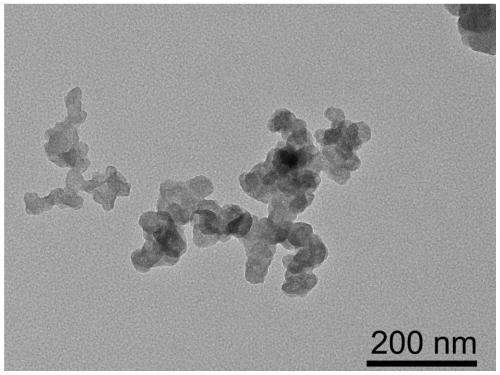
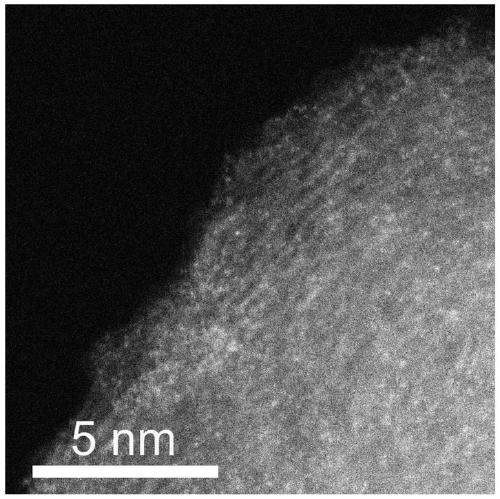
Transition metal single-atom catalyst as well as preparation method and application thereof
InactiveCN109908904AIncrease loadLow priceMetal/metal-oxides/metal-hydroxide catalystsElectrodesFaraday efficiencyPhenanthroline
The invention provides a transition metal single-atom catalyst. In the catalyst, transition metal single atoms are taken as active ingredients, carbon black is taken as a carrier, the loading capacityof the transition metal single atoms is 0.5-6wt%, and the highest loading capacity of the transition metal single atoms is higher than that of the prior art by 2-3 times. Various low-price transitionmetal single-atom catalysts with high dispersibility and high loading capacity are formed by inducing a limit range and by taking phenanthroline with strong complexation effect as a ligand and takingthe carbon black with high conductivity and high surface activity as the carrier. A preparation method of the transition metal single-atom catalyst has universality and can be used for preparing various low-price transition metal single-atom catalysts; according to the method, a Cr single-atom catalyst, an Nb single-atom catalyst, an Rh single-atom catalyst, a Cd single-atom catalyst, an Os single-atom catalyst and an Ir single-atom catalyst are prepared for the first time. Additionally, the preparation method has the advantages of being low in cost and simple in process and achieving large-scale production. Next, the catalyst has higher selectivity and Faraday efficiency in application of preparation of carbon monoxide by reducing carbon dioxide through electro-catalysis.
Owner:TECHNICAL INST OF PHYSICS & CHEMISTRY - CHINESE ACAD OF SCI
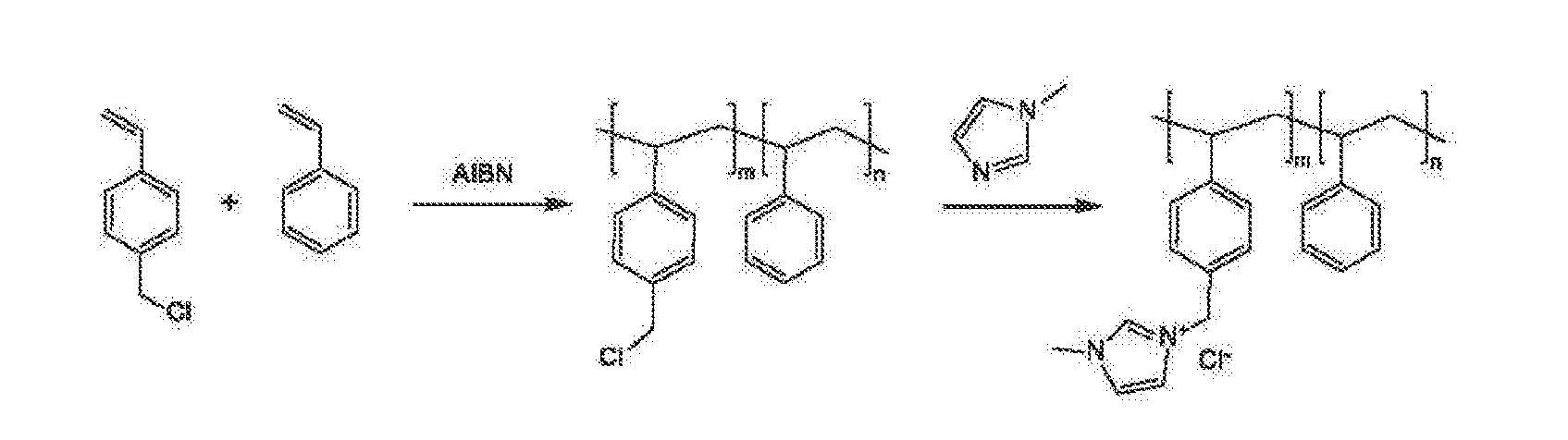
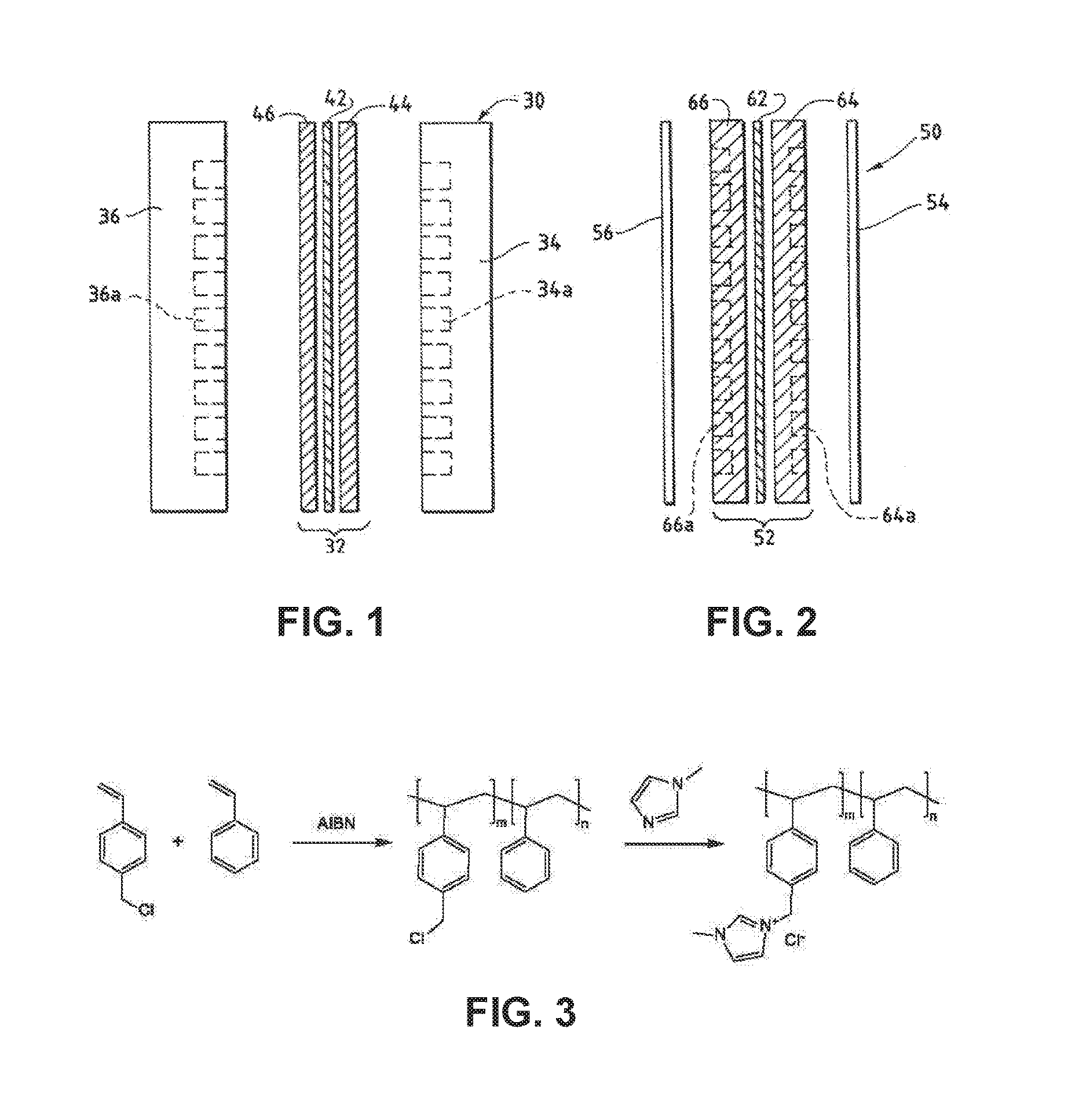
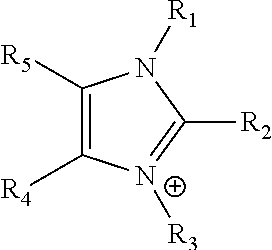
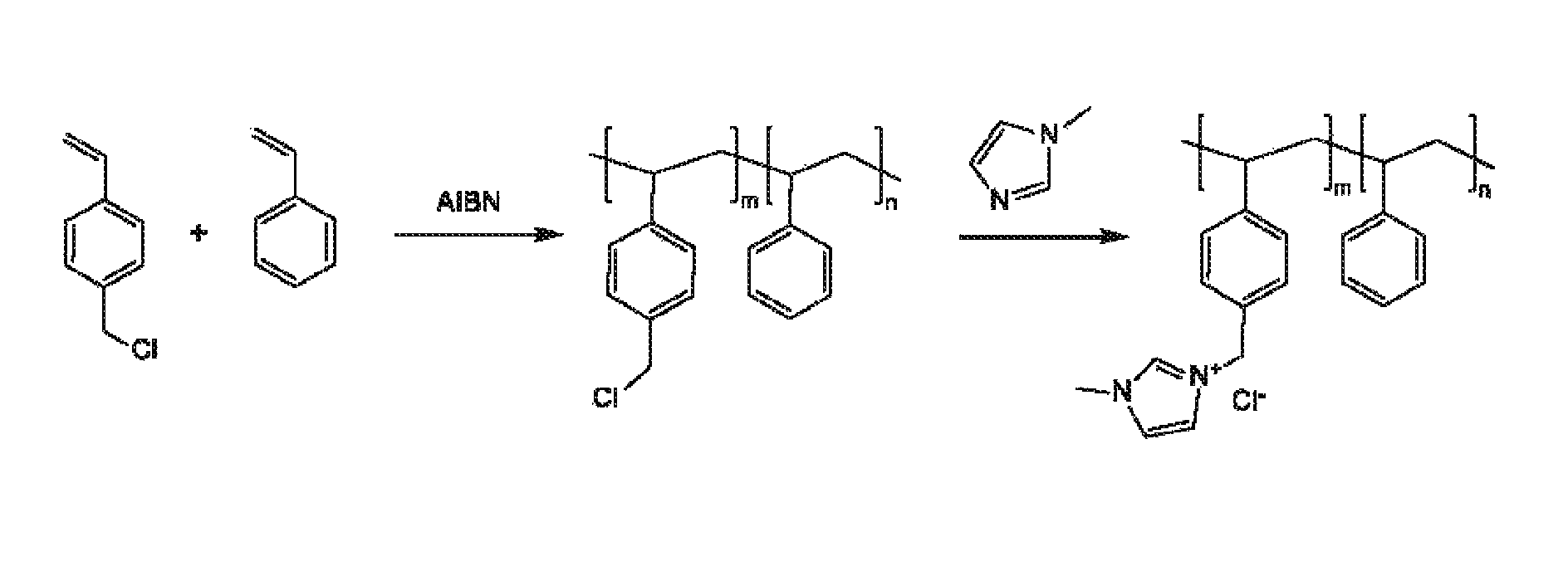
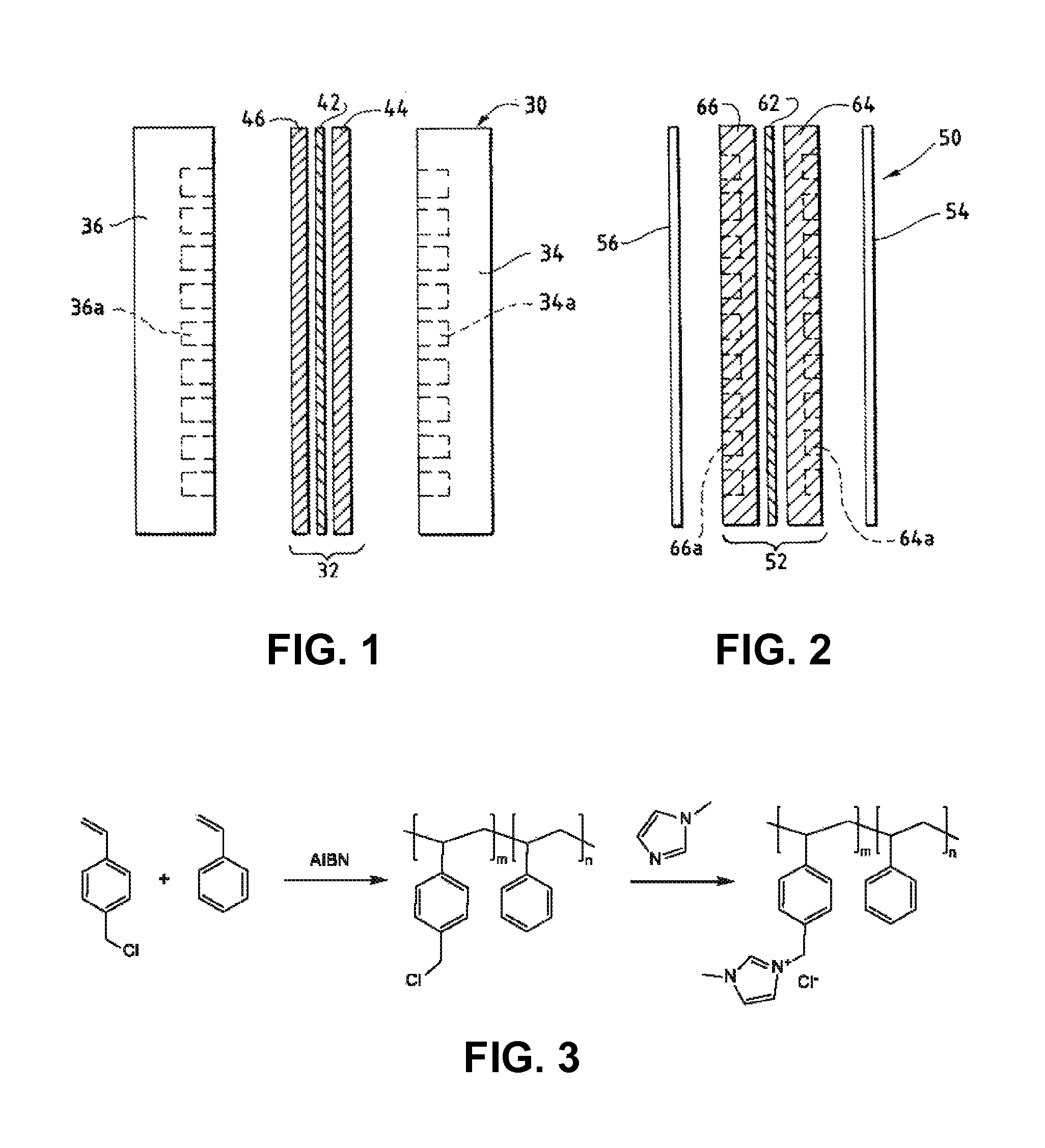
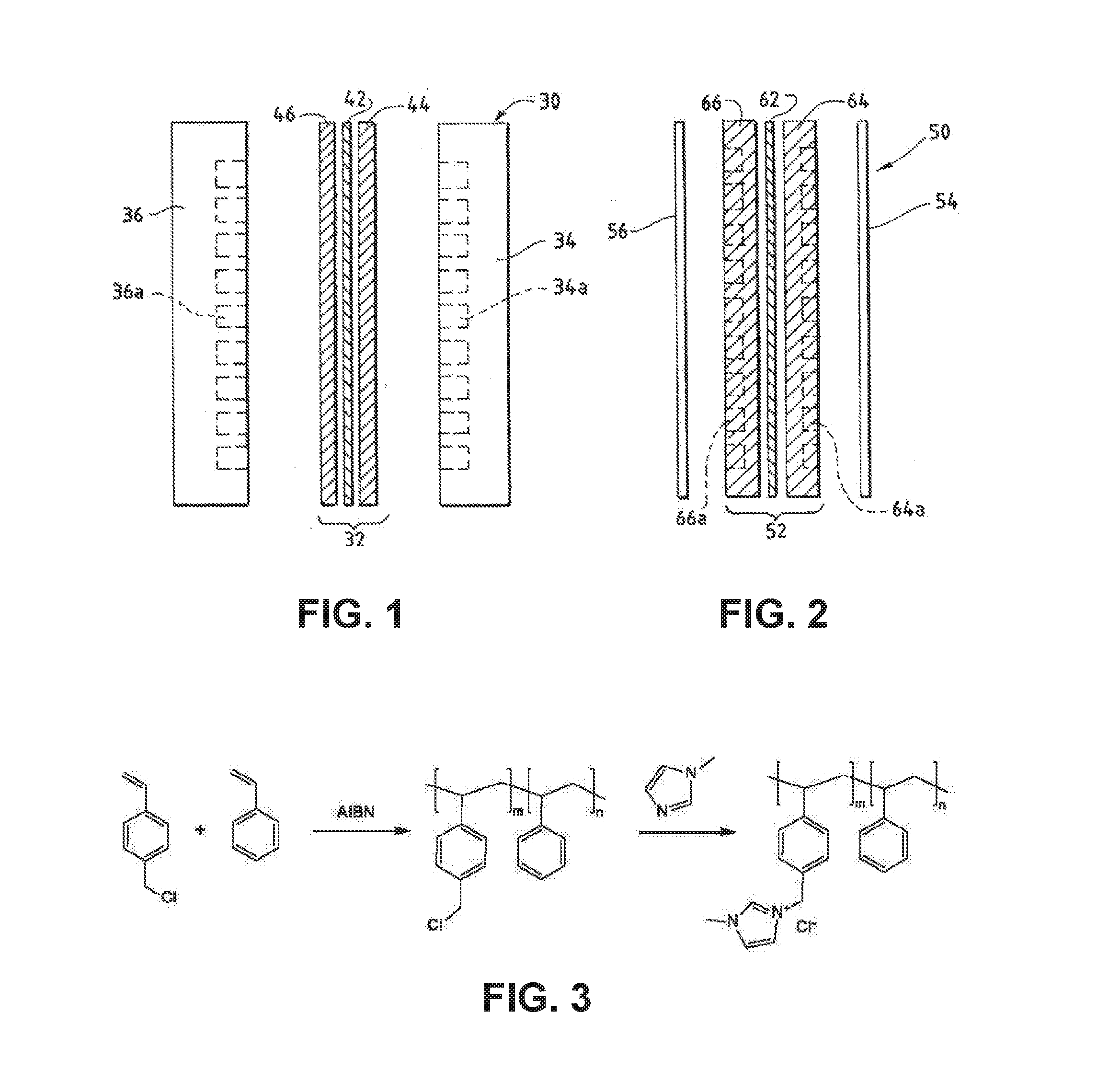
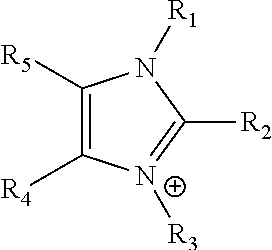
Ion-conducting membranes
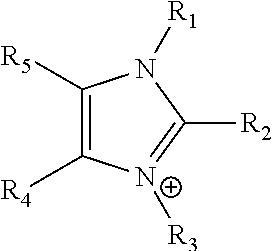
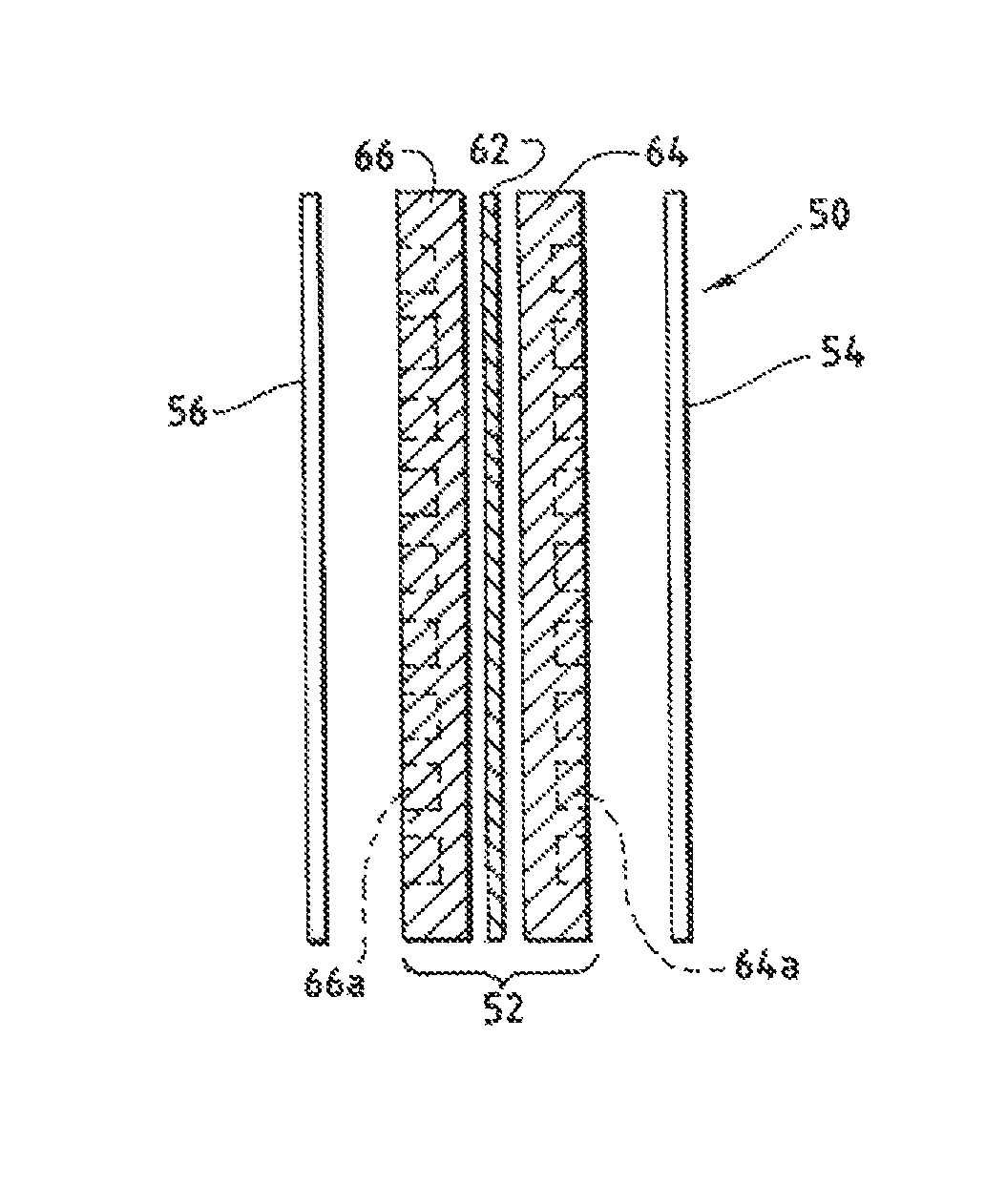
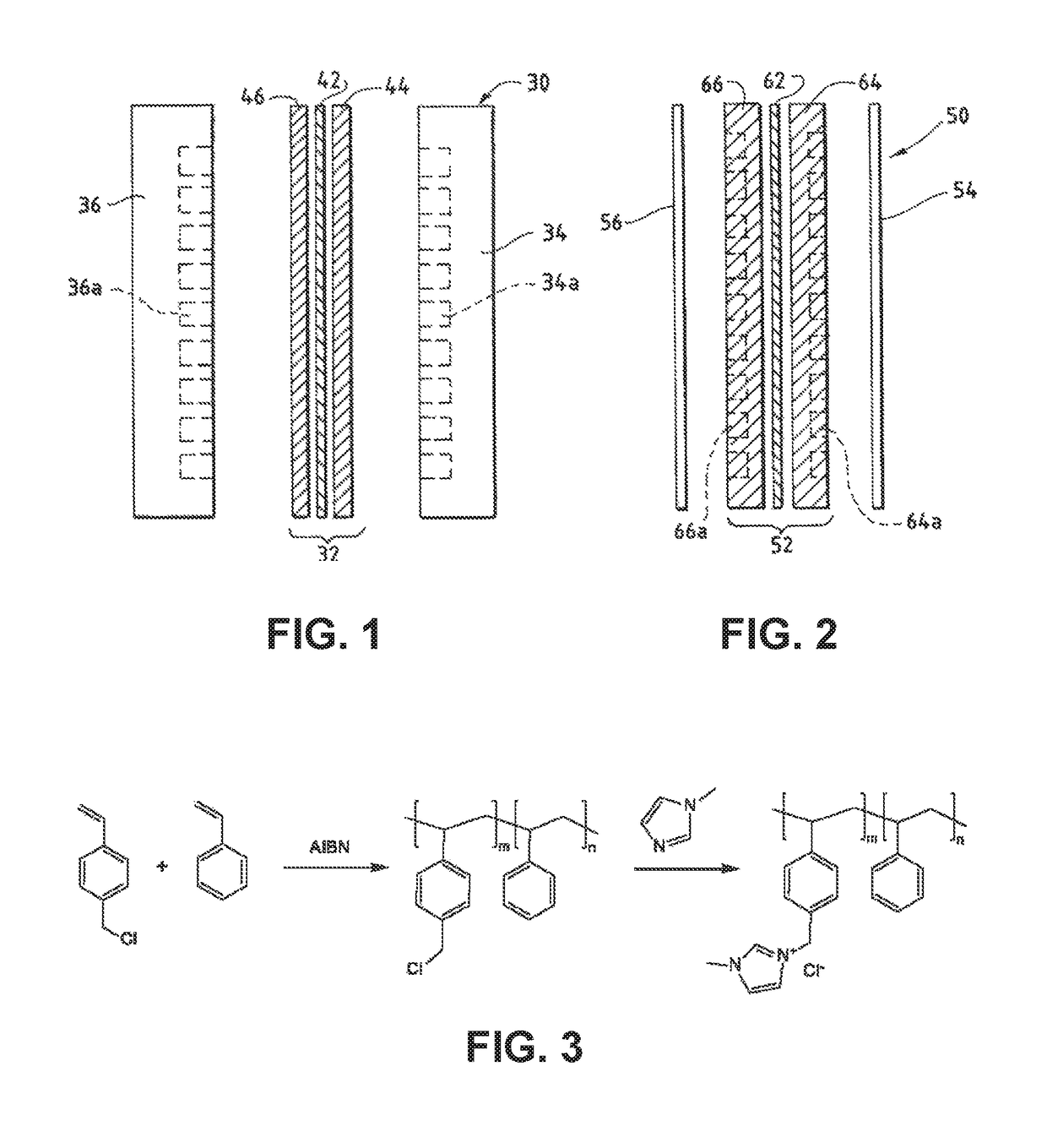
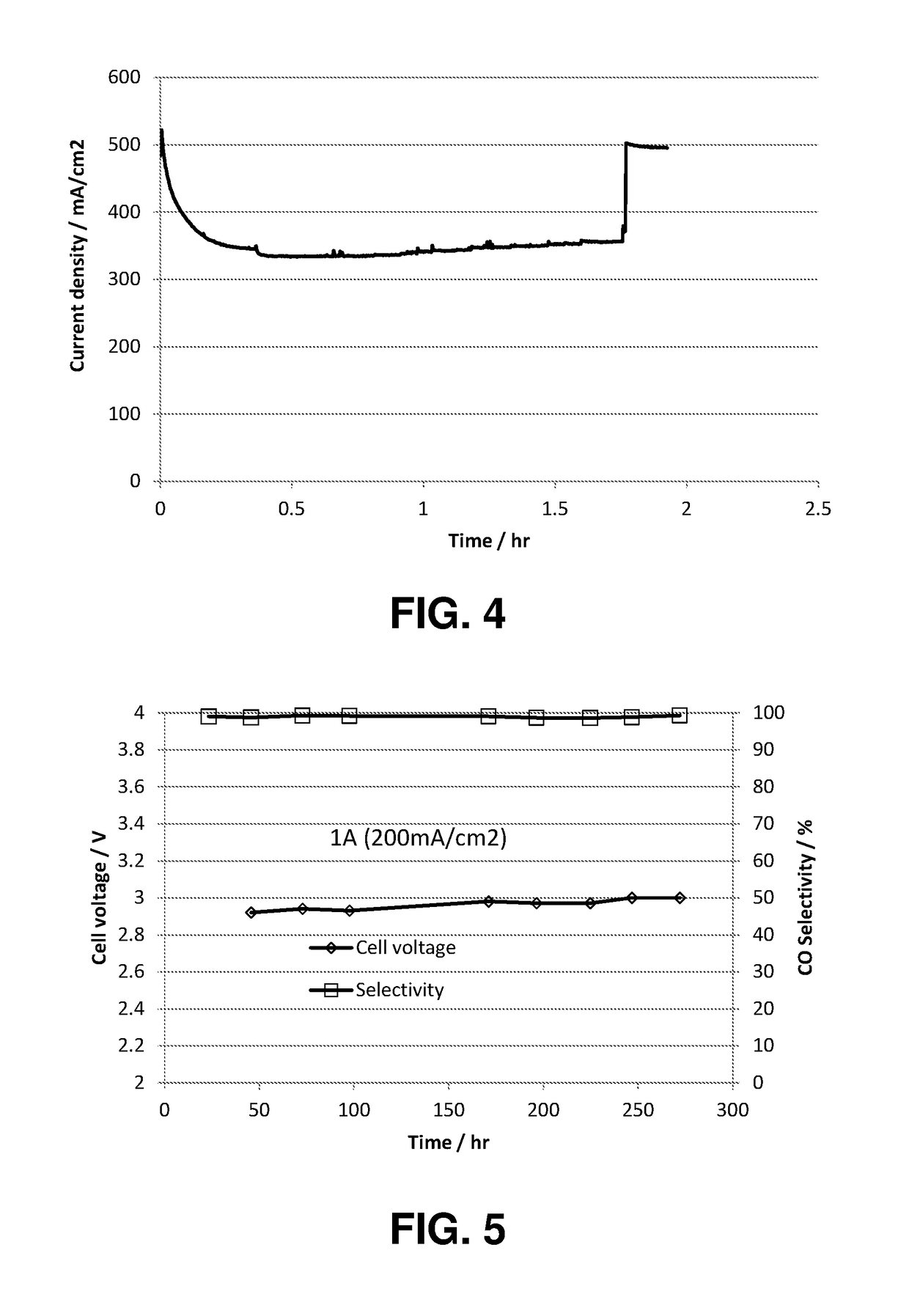
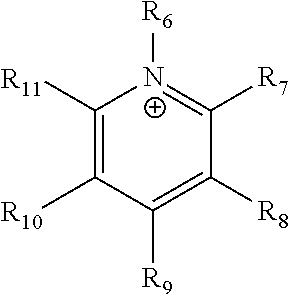
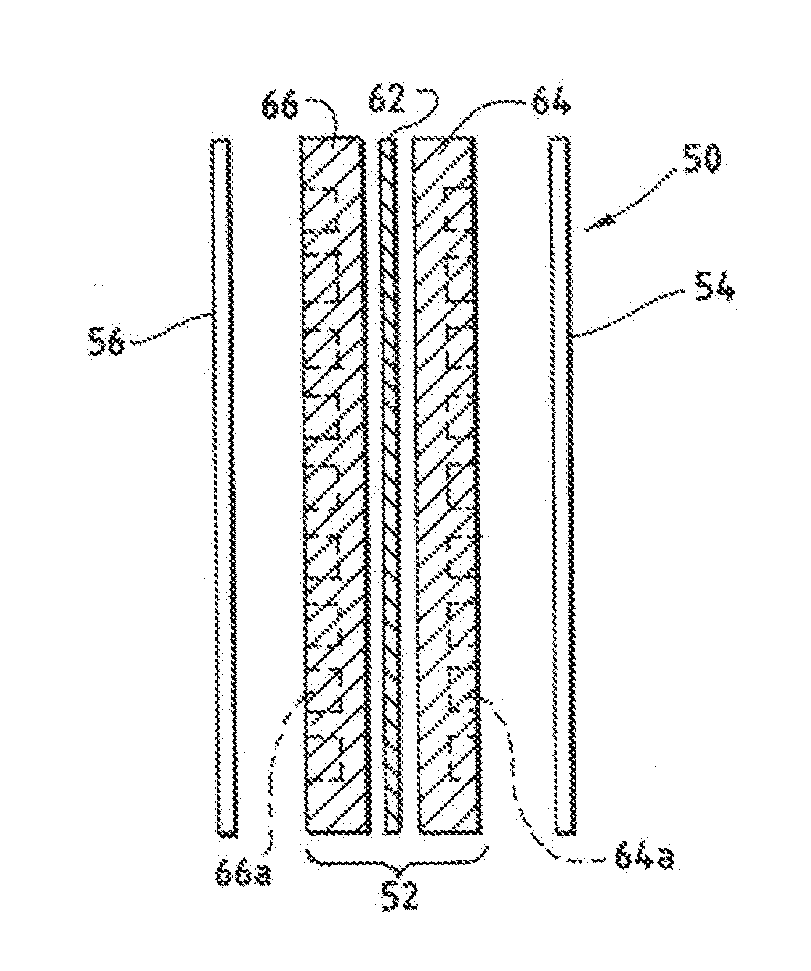
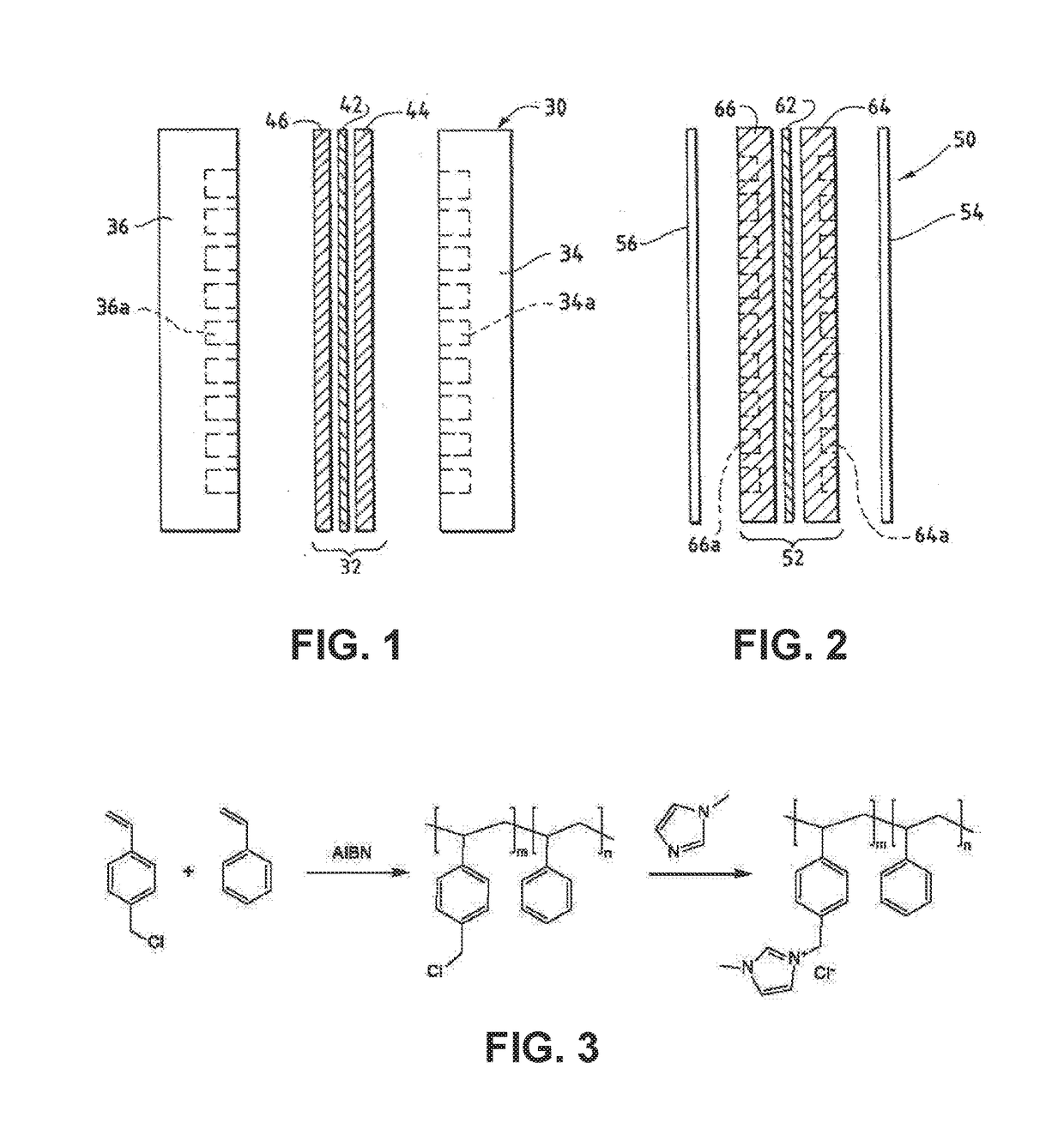
An ion conducting polymeric composition mixture comprises a copolymer of styrene and vinylbenzyl-Rs. Rs is selected from the group consisting of imidazoliums, pyridiniums, pyrazoliums, pyrrolidiniums, pyrroliums, pyrimidiums, piperidiniums, indoliums, and triaziniums. The composition contains 10%-90% by weight of vinylbenzyl-Rs. The composition can further comprise a polyolefin comprising substituted polyolefins, a polymer comprising cyclic amine groups, a polymer comprising at least one of a phenylene group and a phenyl group, a polyamide, and / or the reaction product of a constituent having two carbon-carbon double bonds. The composition can be in the form of a membrane. In a preferred embodiment, the membrane is a Helper Membrane that increases the faradaic efficiency of an electrochemical cell into which the membrane is incorporated, and also allows product formation at lower voltages than in cells without the Helper Membrane.
Owner:DIOXIDE MATERIALS
Ion-conducting membranes
An ion conducting polymeric composition mixture comprises a copolymer of styrene and vinylbenzyl-Rs. Rs is selected from the group consisting of imidazoliums and pyridiniums. The composition contains 10%-90% by weight of vinylbenzyl-Rs. The composition can further comprise a polyolefin comprising substituted polyolefins, a polymer comprising cyclic amine groups, a polymer comprising at least one of a phenylene group and a phenyl group, a polyamide, and / or the reaction product of a constituent having two carbon-carbon double bonds. The composition can be in the form of a membrane. In a preferred embodiment, the membrane is a Helper Membrane that increases the faradaic efficiency of an electrochemical cell into which the membrane is incorporated, and also allows product formation at lower voltages than in cells without the Helper Membrane.
Owner:DIOXIDE MATERIALS
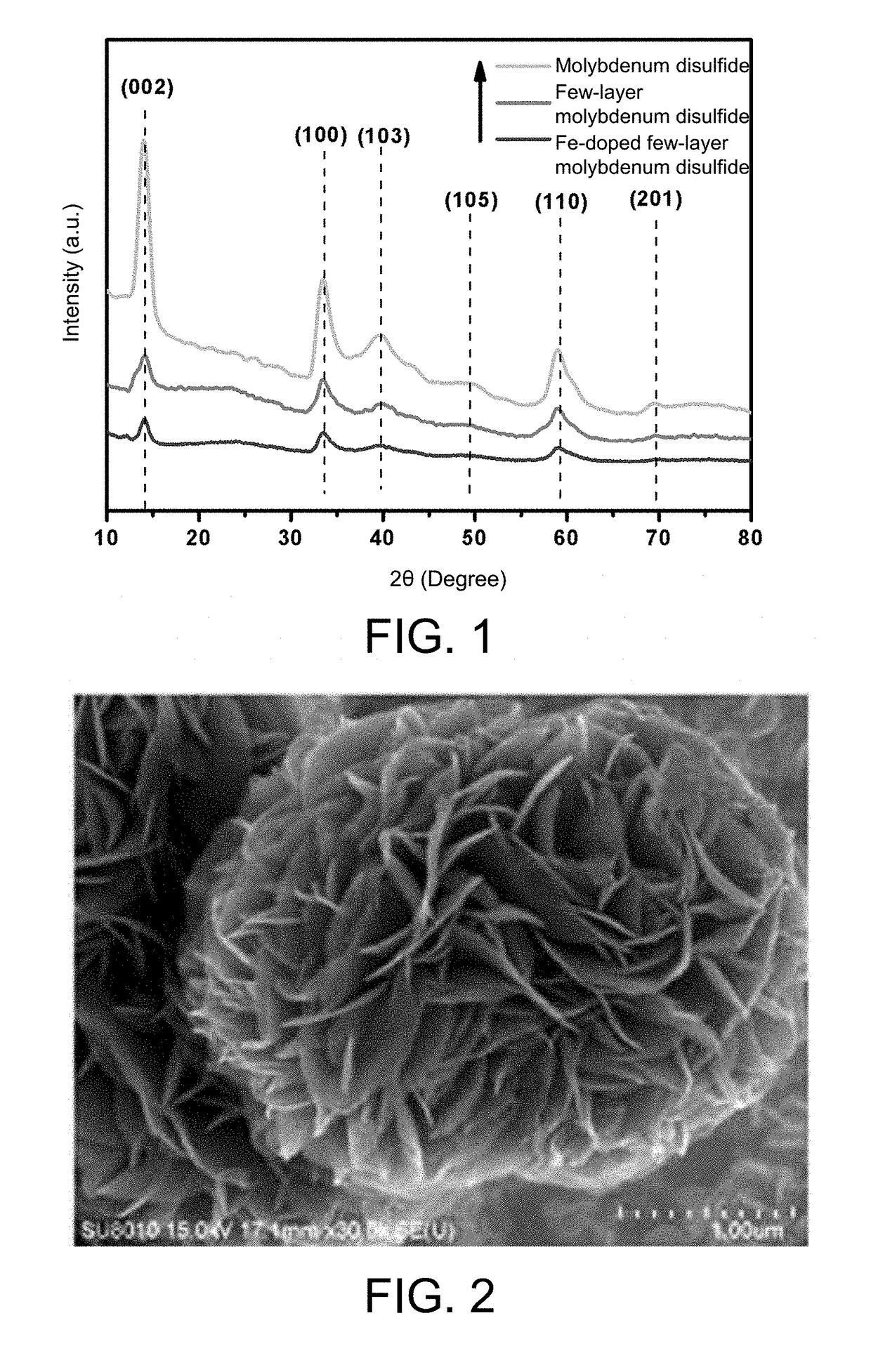
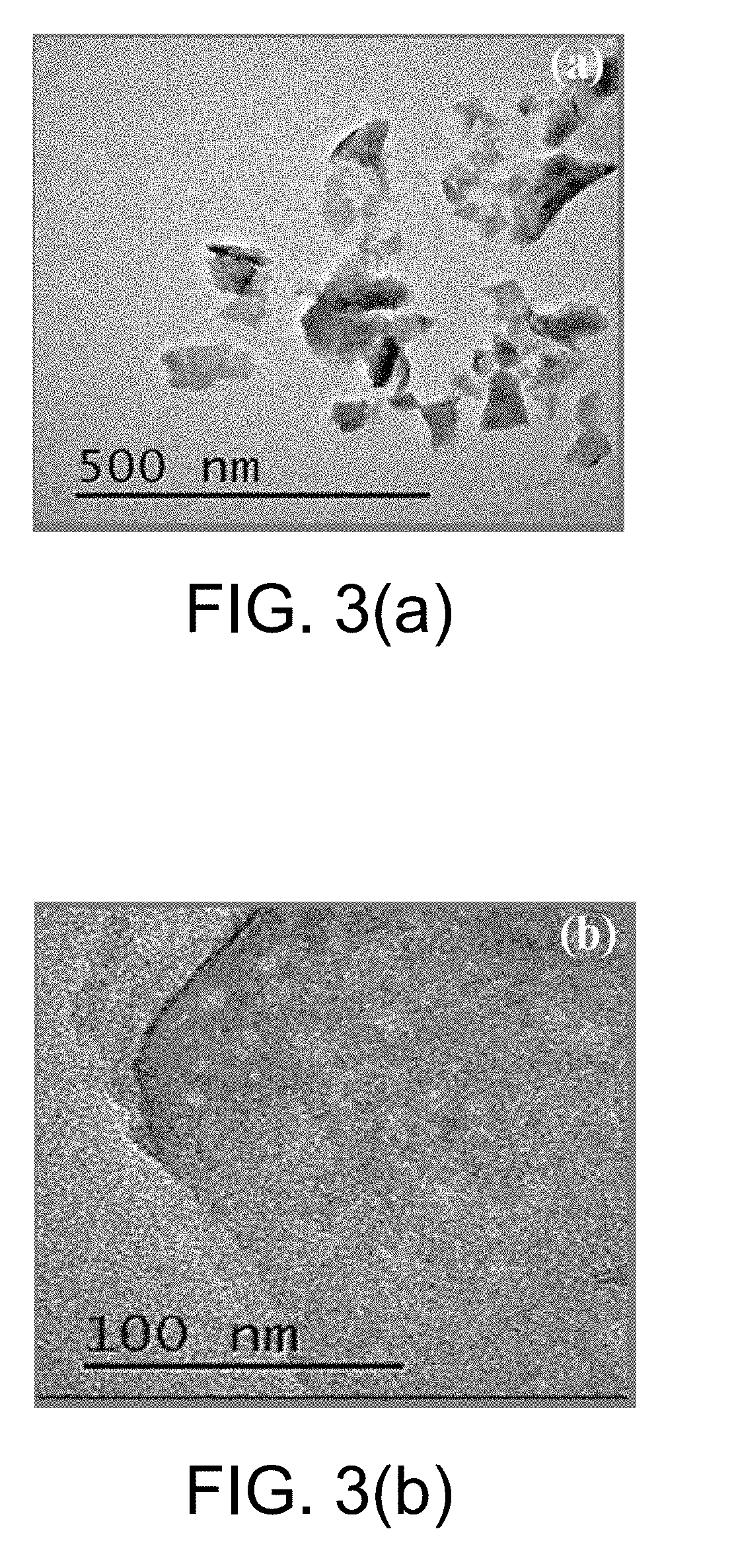
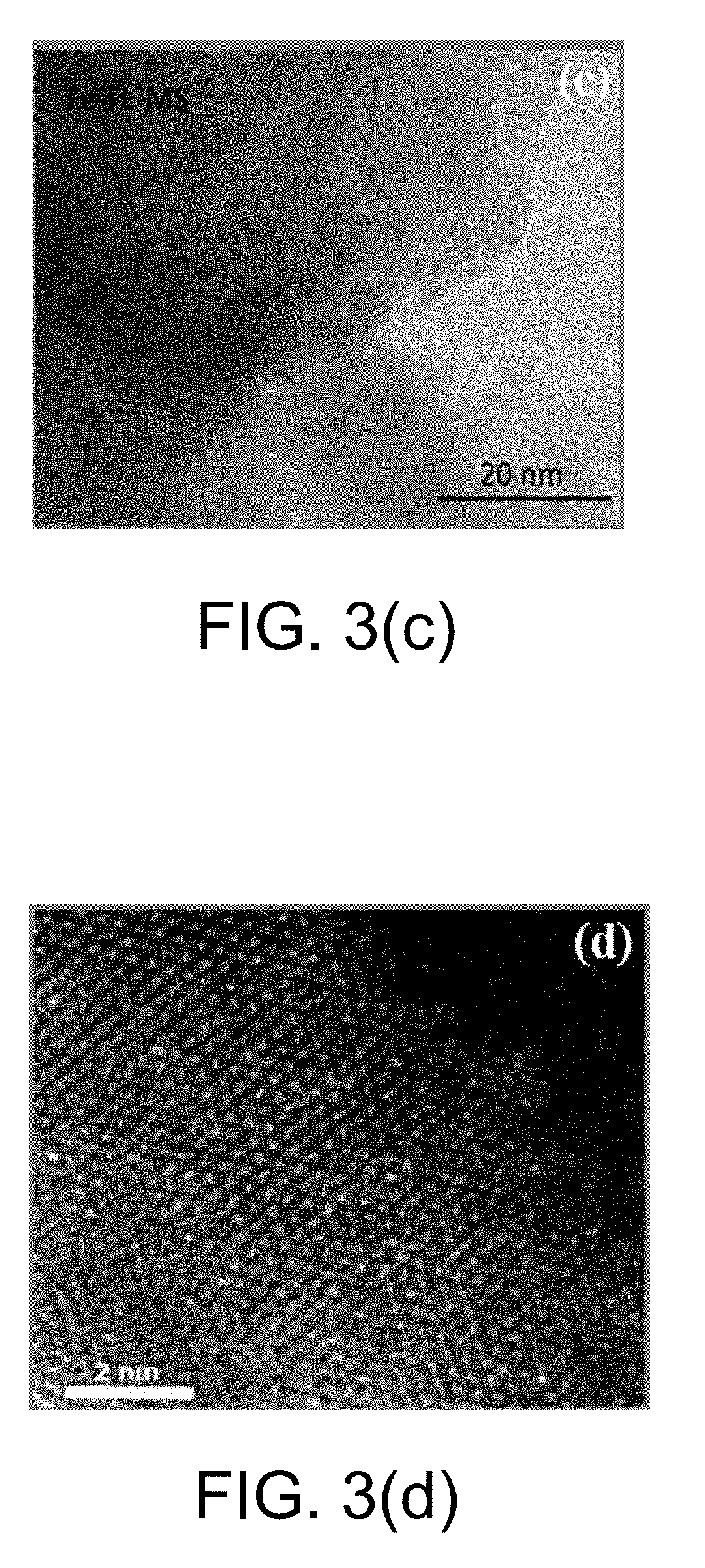
Monatomic metal-doped few-layer molybdenum disulfide electrocatalytic material, preparing method thereof, and method for electrocatalytic nitrogen fixation
ActiveUS20190030516A1Readily availableLow costHeterogenous catalyst chemical elementsCatalyst activation/preparationFaraday efficiencySelective reduction
The present invention provides a monatomic metal-doped few-layer molybdenum disulfide electrocatalytic material, a preparing method thereof, and a method for electrocatalytic nitrogen fixation. The material has a few-layer ultra-thin and irregular flake-like microstructure with a length and a width of nanometer scale. A doping metal in the monatomic metal-doped few-layer molybdenum disulfide electrocatalytic material is dispersed in a form of single atoms. When the catalyst is used in electrochemical reduction of N2, a Faradic efficiency in selective reduction of N2 into NH4+ is 18% or above, and stability of the catalyst is better.
Owner:HUAZHONG NORMAL UNIV
Ion-conducting membranes
An anion-conducting polymeric membrane comprises a terpolymer of styrene, vinylbenzyl-Rs and vinylbenzyl-Rx. Rs is a positively charged cyclic amine group. Rx is at least one constituent selected from the group consisting Cl, OH and a reaction product between an OH or Cl and a species other than a simple amine or a cyclic amine. The total weight of the vinylbenzyl-Rx groups is greater than 0.3% of the total weight of the membrane. In a preferred embodiment, the membrane is a Helper Membrane that increases the faradaic efficiency of an electrochemical cell into which the membrane is incorporated, and also allows product formation at lower voltages than in cells without the Helper Membrane.
Owner:DIOXIDE MATERIALS
Electrode for electrochemical reduction of CO2 and preparation of formic acid and preparation method and application thereof
ActiveCN104846397AIncrease current densityLow costElectrolytic organic productionElectrode shape/formsFaraday efficiencyReaction rate
The invention relates to an electrode for electrochemical reduction of CO2 and preparation of formic acid and a preparation method and an application thereof, and belongs to the field of a carbon dioxide resource technology. A traditional metal electrode has smooth surface and few catalytic active sites, which are not beneficial to rapid reaction. According to the invention, foamy copper with high specific surface is used as a substrate, and a layer of a low-melting-point metal catalyst is electroplated on the surface of the foamy copper so as to effectively raise reaction rate. The foamy copper can undergo surface electroplating in different formulations of electroplate liquids. The electroplated electrode still maintains a porous structure of foamy copper. The low-melting-point metal coating on the surface can efficiently electro-catalyze carbon dioxide to prepare formic acid. By the method, insufficiency of low surface catalytic active point of a traditional metal electrode can be improved. High Faradic efficiency of electrochemical reduction of CO2 and preparation of formic acid is guaranteed, and generation speed of formic acid also can be accelerated. The method is expected to be applied in the industrial process.
Owner:日照新睿招商发展有限公司
Ion-Conducting Membranes
An ion conducting polymeric composition mixture comprises a copolymer of styrene and vinylbenzyl-Rs. Rs is selected from the group consisting of imidazoliums, pyridiniums, pyrazoliums, pyrrolidiniums, pyrroliums, pyrimidiums, piperidiniums, indoliums, and triaziniums. The composition contains 10%-90% by weight of vinylbenzyl-Rs. The composition can further comprise a polyolefin comprising substituted polyolefins, a polymer comprising cyclic amine groups, a polymer comprising at least one of a phenylene group and a phenyl group, a polyamide, and / or the reaction product of a constituent having two carbon-carbon double bonds. The composition can be in the form of a membrane. In a preferred embodiment, the membrane is a Helper Membrane that increases the faradaic efficiency of an electrochemical cell into which the membrane is incorporated, and also allows product formation at lower voltages than in cells without the Helper Membrane.
Owner:DIOXIDE MATERIALS
Alloy nanoparticles, preparation method and applications thereof
ActiveCN110560081AImprove catalytic performanceRich varietyMetal/metal-oxides/metal-hydroxide catalystsElectrodesNano catalystFaraday efficiency
The invention discloses alloy nanoparticles, a preparation method and applications thereof. According to the method, a metal precursor loaded on a substrate is rapidly reduced at a high temperature, wherein the metal is rapidly nucleated to avoid the generation of the split-phase alloy so as to form the alloy nanoparticles with ultra-small particle size; and by controlling the types of the metal salts in the precursor, the components in the alloy nanoparticles can be effectively regulated. According to the present invention, the FeCoPtPdIr@GO (FeCoPtPdIr alloy particles are loaded on the surface of graphene oxide) prepared in the embodiments of the invention shows excellent electrochemical hydrolysis hydrogen production performance, can stably operate for 150 h under a condition of 10 mA.cm<-2>, has excellent electrochemical stability, and has the Faraday efficiency of 99.4%, wherein the eta 10 of the product of the present invention is equal to 42 mV, and far exceeds the eta 10 of thecommercial Pt / C of 64 mV (the smaller the eta 10, the better the electrochemical hydrolysis hydrogen production performance); and the new thought is provided in the preparation of alloy nanoparticlesand alloy nanometer catalysts, and the development of alloy nanometer catalysts in catalysis and energy is promoted.
Owner:ZHEJIANG UNIV
Metal monoatomic catalyst loaded by flexible carbon-based carrier and preparation method and application of catalyst
ActiveCN110102300AEasy to prepareMild conditionsMetal/metal-oxides/metal-hydroxide catalystsElectrodesFaraday efficiencyHeteroatom
The invention discloses a metal monoatomic catalyst loaded by a flexible carbon-based carrier and a preparation method and application of the catalyst, and belongs to the technical field of electrocatalysis. The catalyst is formed by coordinating and anchoring metal monoatoms and heteroatoms on graphene and loading the graphene on the flexible carbon-based carrier. The catalyst is prepared from, by weight, 0.001-4.0% of the metal monoatoms, 6-34.999% of the graphene, 50-80% of the carbon-based carrier and 5-20% of the heteroatoms. The preparation method of the metal monoatomic catalyst loadedby the flexible carbon-based carrier is simple, the content of the metal monoatoms is high, the metal monoatoms are dispersed uniformly and anchored on the carrier through coordination with the heteroatoms, and the physicochemical structure is stable. The catalyst is applied to electrocatalytic reduction of CO2 and has excellent CO selectivity, high CO Faraday efficiency and good recycling stability.
Owner:ZHONGBEI UNIV
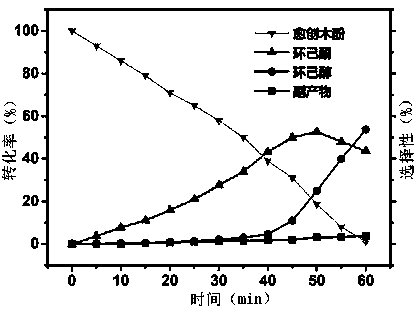
Method for preparing KA oil and derivatives of KA oil by electrocatalytic hydrogenation of lignin-based phenolic compounds
ActiveCN108660479AImprove conversion rateGood choiceElectrolytic organic productionChemical recyclingElectricityPlatinum
The invention relates to a method for preparing KA oil and derivatives by electrocatalytic hydrogenation of lignin-based phenolic compounds. According to the method, an H-shaped electrolytic cell is used as a container, in a negative pole chamber of the electrolytic cell, carbon fiber cloth is coated with a supported composite catalyst as a working electrode, and the lignin-based phenolic compounds are used as reaction substrates to be dissolved in an acidic solution as a negative pole solution; and a positive pole chamber uses a platinum sheet as a counter electrode and the acidic solution aspositive pole liquid, an electrocatalytic hydrogenation reaction is carried out at the temperature of 30-90 DEG C for 0.5-2 hours, and the KA oil and the derivatives of the KA oil are obtained afterpost-treatment. By the adoption of the method, by adopting the composite catalyst, the service life of the catalyst is greatly prolonged, and the conversion rate of the lignin-based phenolic compoundsreaches over 90-99%, the selectivity of the KA oil and the derivatives of the KA oil reaches over 90-95%, the Faraday efficiency can reach 80-90%, the cost is low, environmental protection is realized, the technological process is simple, the supported composite catalyst is recyclable, the production cost lowered, and the method has the high industrial value.
Owner:ZHEJIANG UNIV OF TECH
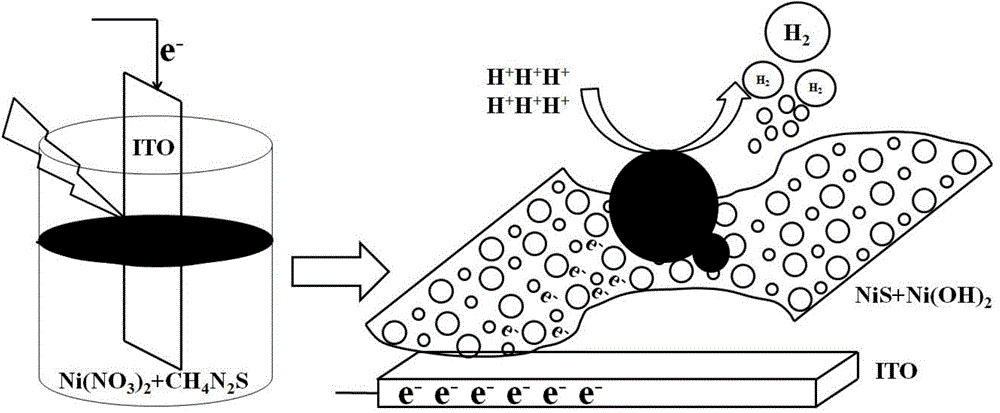
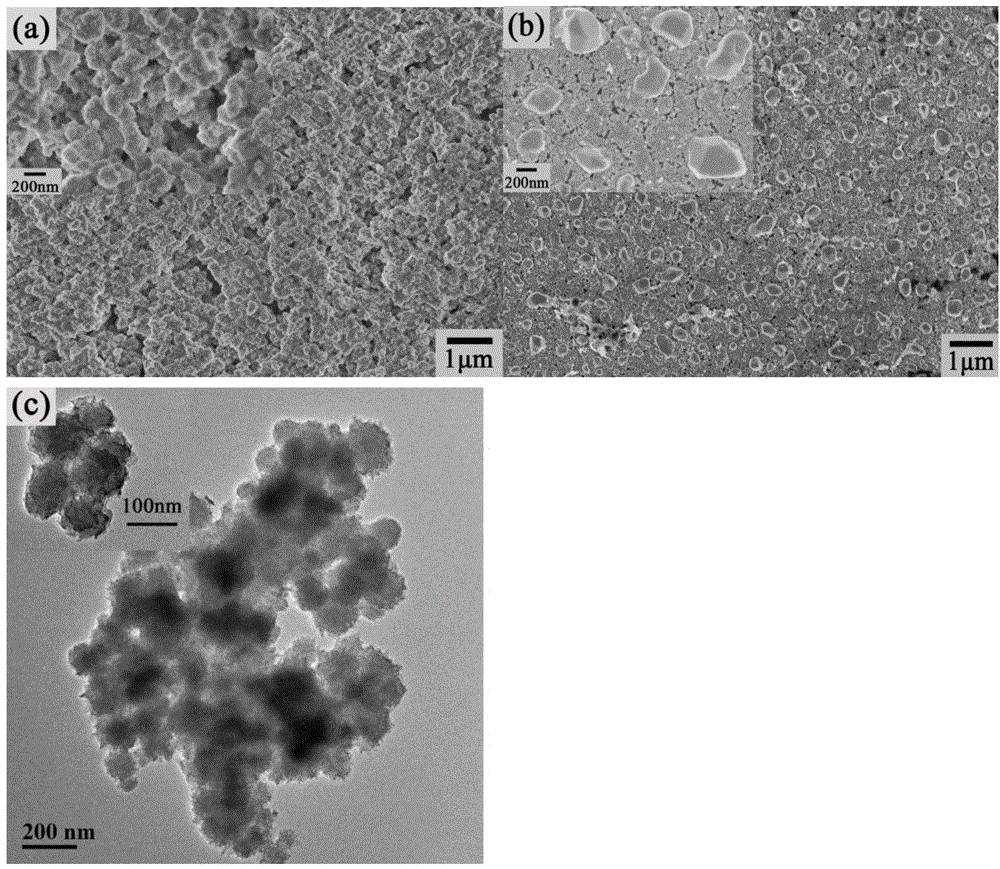
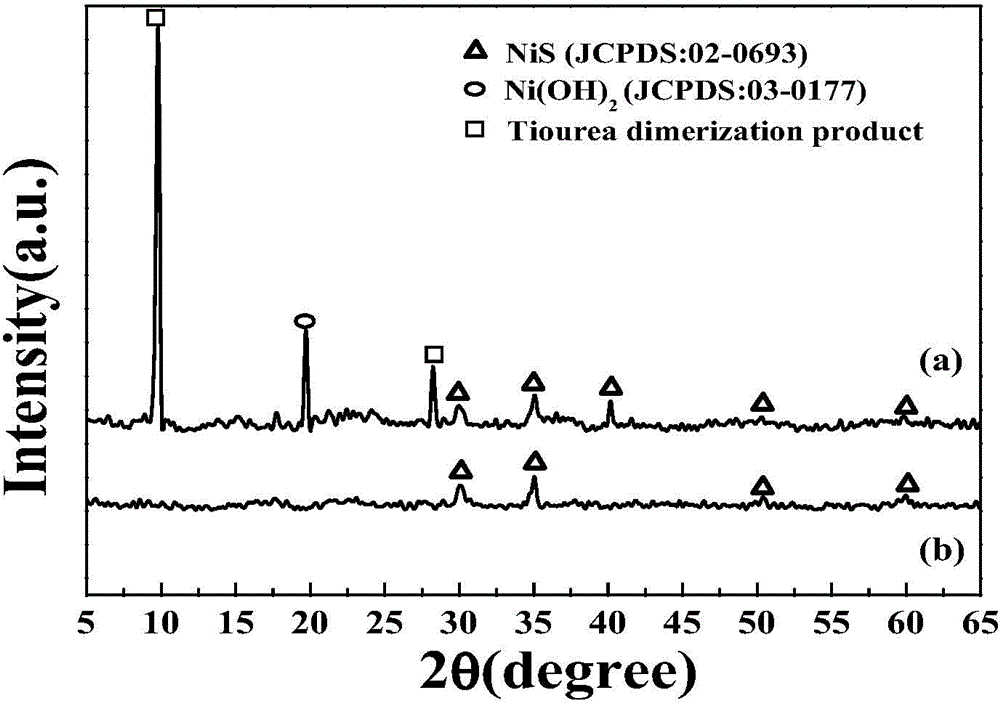
Method for preparing NiS/Ni(OH)2 electrocatalyst used for decomposing water to generate hydrogen
ActiveCN104862758ASimple manufacturing methodEfficient responseElectrolytic inorganic material coatingElectrodesElectricityHydrogen
The invention discloses a method for preparing an NiS / Ni(OH)2 electrocatalyst used for decomposing water to generate hydrogen. The method comprises the steps that nickel nitrate and thiocarbamide are taken as raw materials, an optical assistance electric deposition method is adopted to obtain an NiS / Ni(OH)2 electrode through one step, and further treatment is not needed. A method for preparing the electrode is controllable in amount of coated catalysts, and the obtained electrode represents the good electrocatalytic activity under the lower overpotential, and is stable in performance; the electrode can continuously react for 22 hours in neutral electrolytes, and the current is not obviously reduced; and the faradic efficiency approaches to 100 percent, and the method can be effectively applied to the field of hydrogen generation through electrocatalysis water decomposition.
Owner:JIANGNAN UNIV
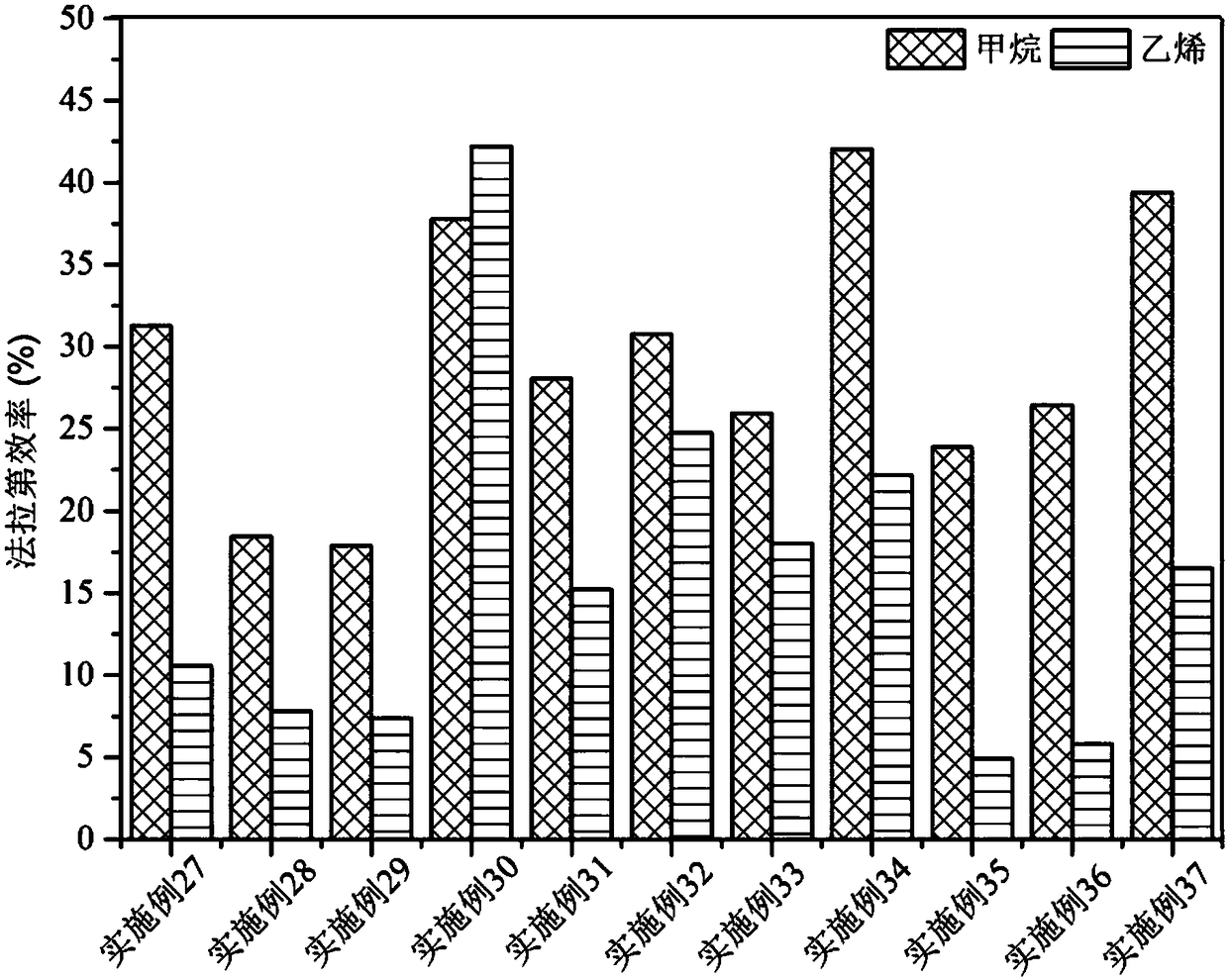
Method for preparing methane and ethylene through carbon dioxide electrochemical reduction
ActiveCN108588748AIncrease the catalytic activity of electrochemical reductionImprove Faraday efficiencyElectrolytic organic productionElectrodesFaraday efficiencyAuxiliary electrode
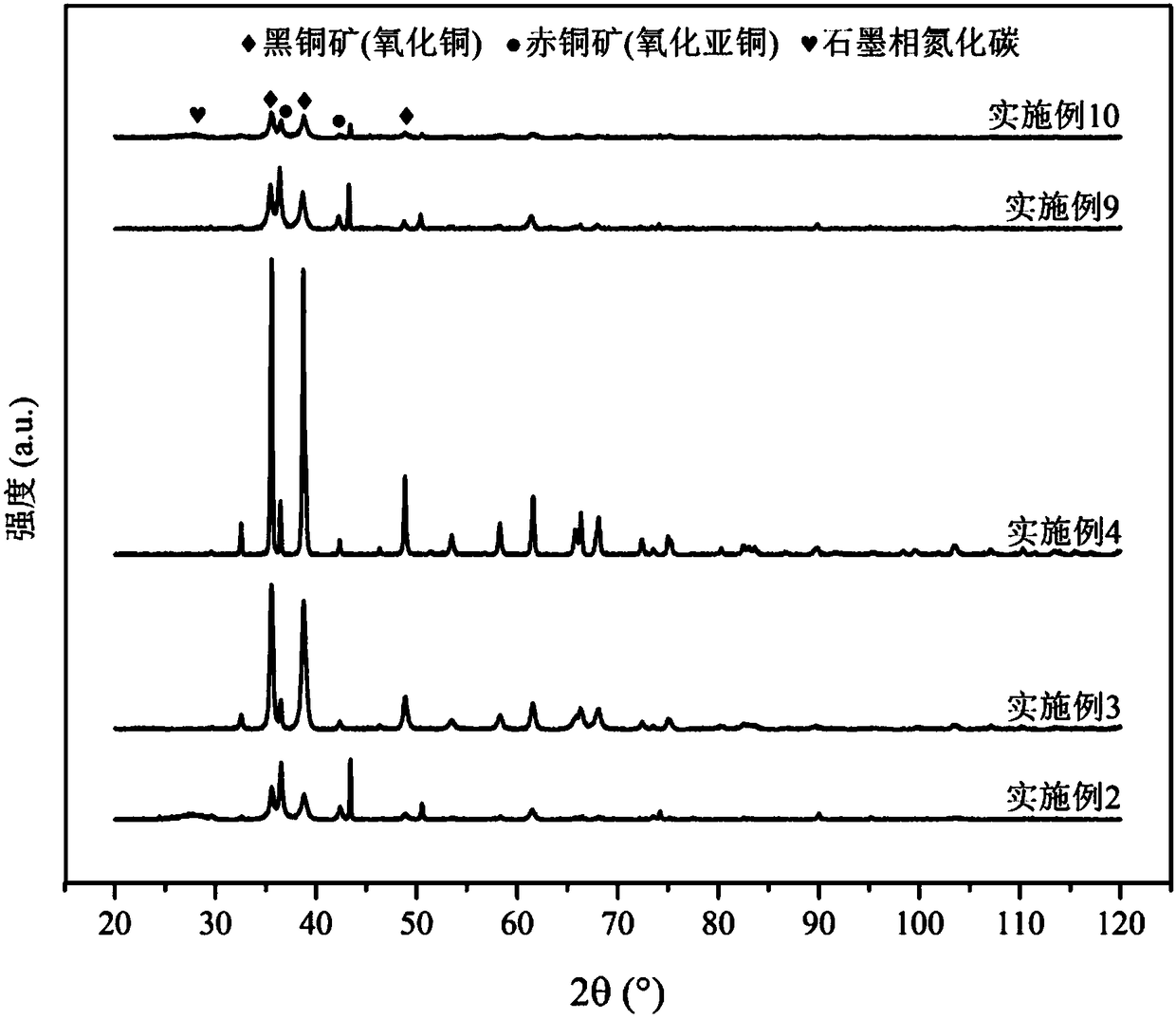
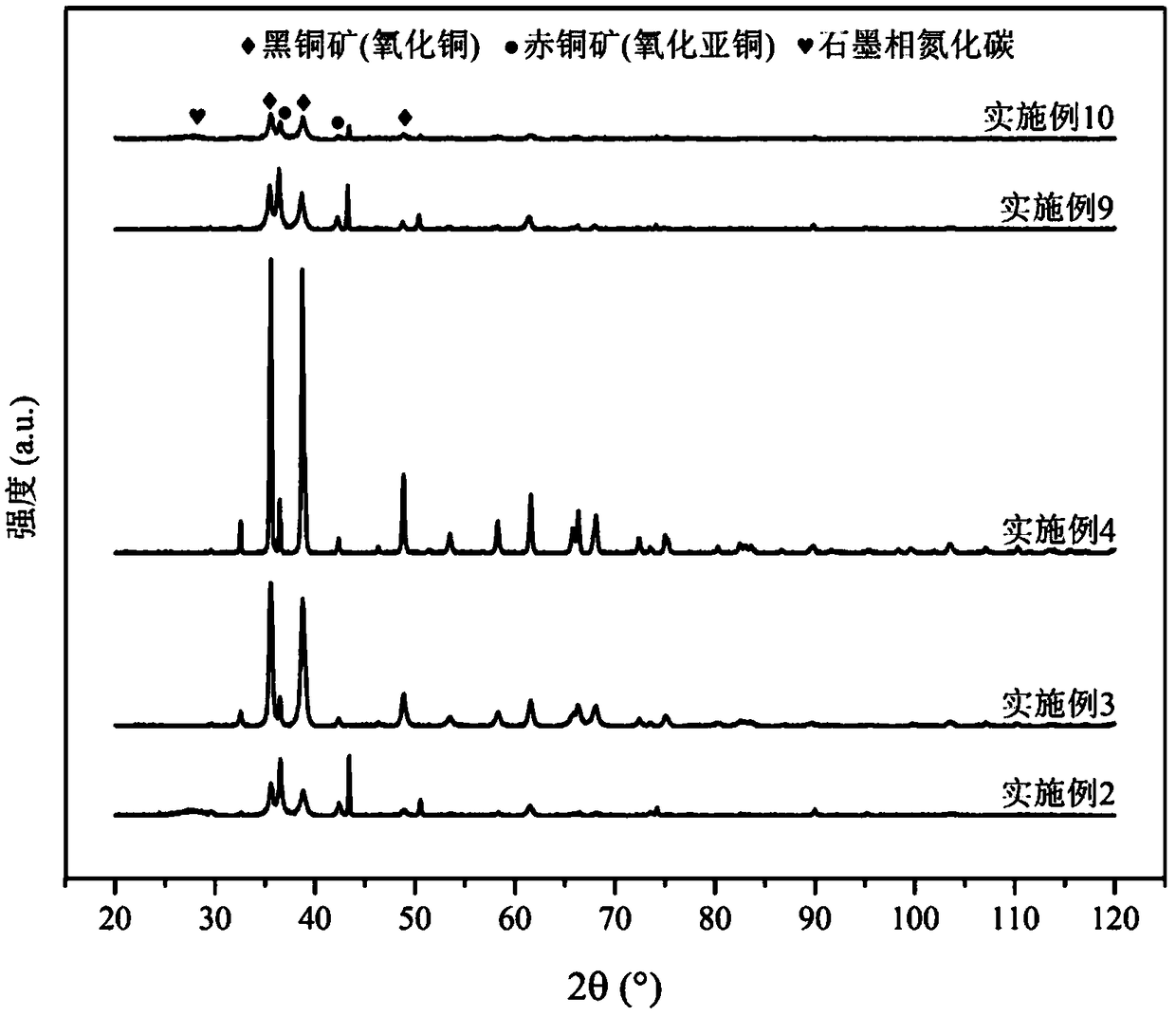
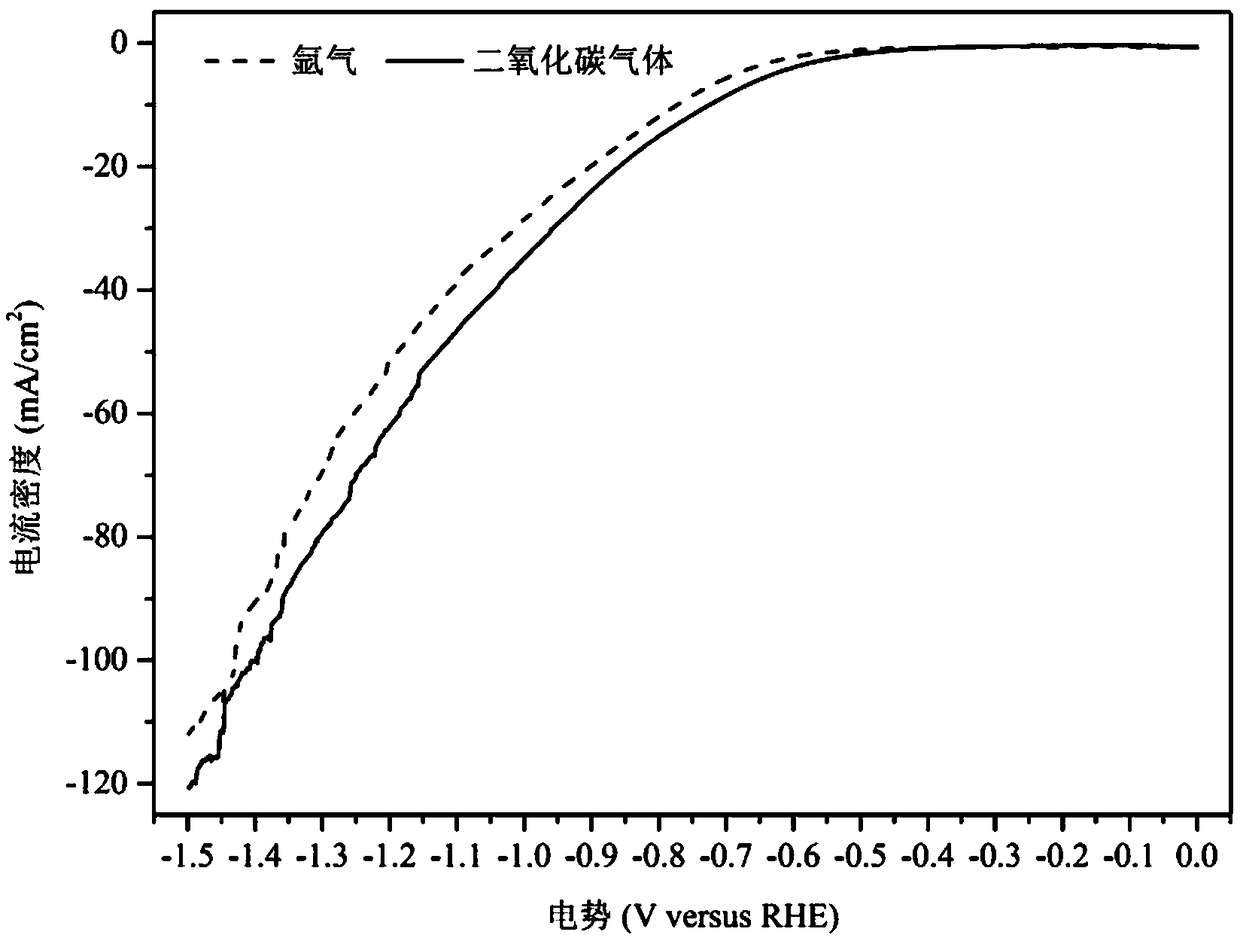
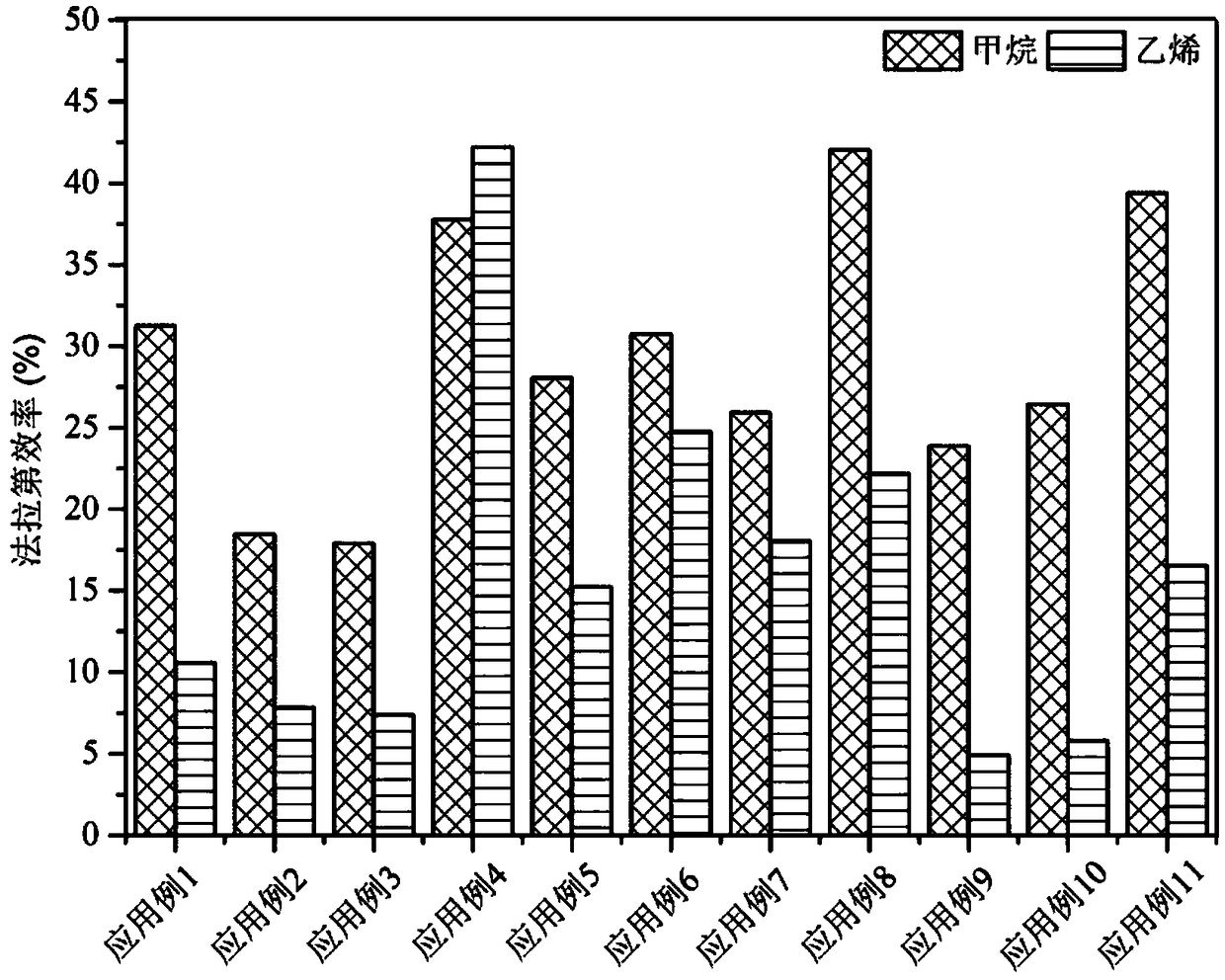
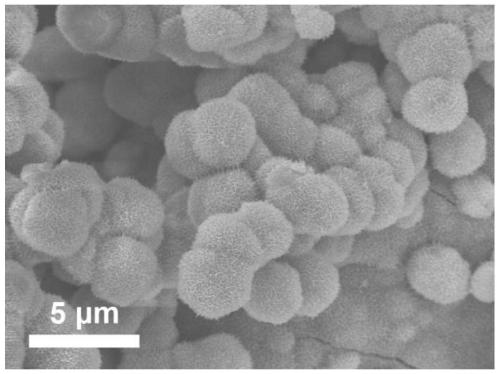
The invention relates to a method for preparing methane and ethylene through carbon dioxide electrochemical reduction. The method comprises the steps that an H-shaped dual-electrochemical-cell reactoris adopted, and is isolated into a cathode chamber and an anode chamber through a proton exchange membrane; before the reaction, carbon dioxide gas is led into the cathode chamber; a three-electrodesystem is adopted, a gas diffusion electrode serves as a work electrode, a platinum electrode serves as an auxiliary electrode, and a silver / silver chloride electrode serves as a reference electrode;the gas diffusion electrode comprises a gas diffusion electrode body and a carbon dioxide electrochemical reduction catalyst loaded gas diffusion electrode body; the carbon dioxide electrochemical reduction catalyst is graphite phase nitrogenated carbon supported nanometer copper oxide, and the nanometer copper oxide has tenorite and red copper ore. Through the method, the faradic efficiency of carbureted hydrogen of methane, ethylene and the like can be improved.
Owner:ZHEJIANG UNIV
Preparation method for loosened porous cuprous oxide material and application of cuprous oxide in electrocatalytic reduction of carbon dioxide
InactiveCN109536991AEasy to synthesizeSynthesis is safe and controllableElectrolytic organic productionElectrodesActivation barrierHigh carbon
Owner:TIANJIN UNIV
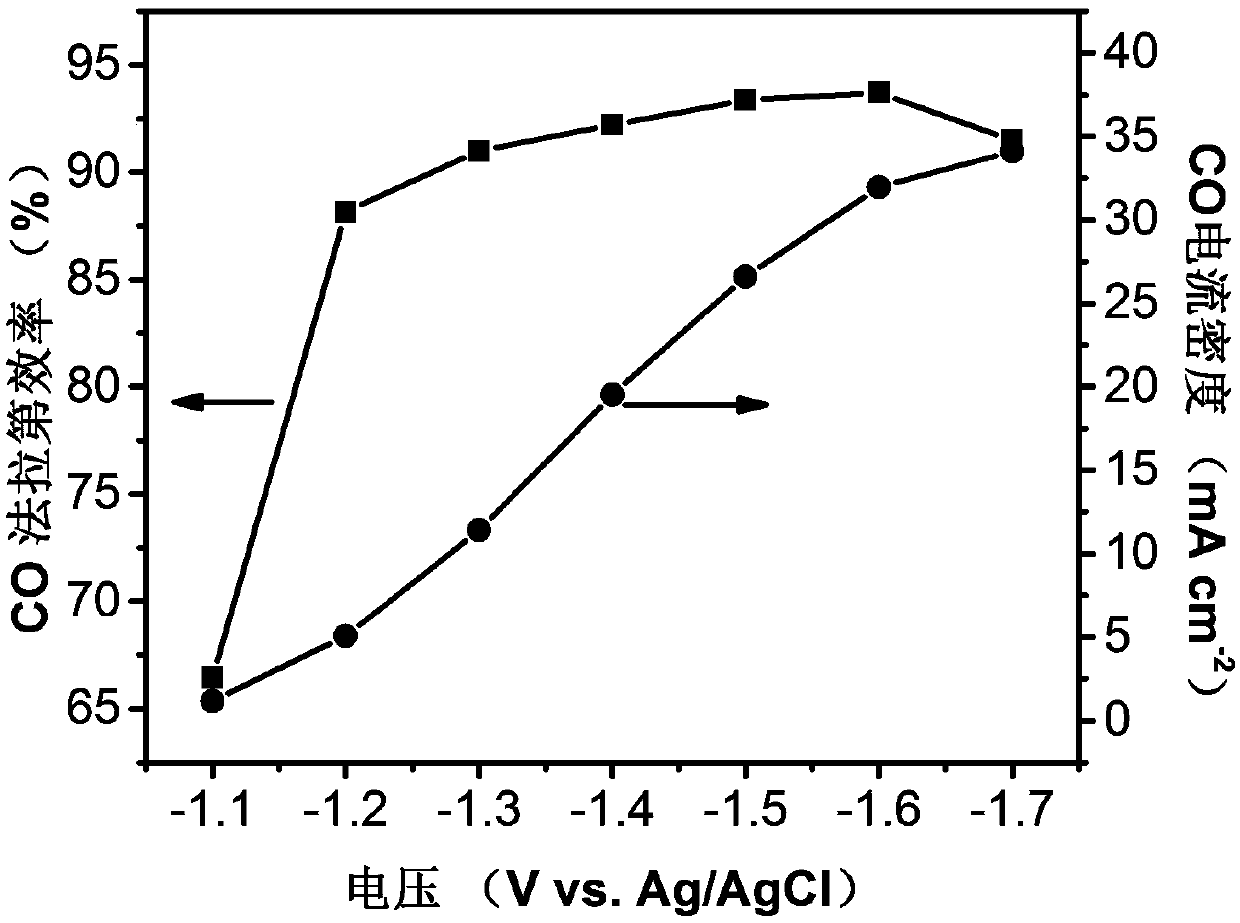
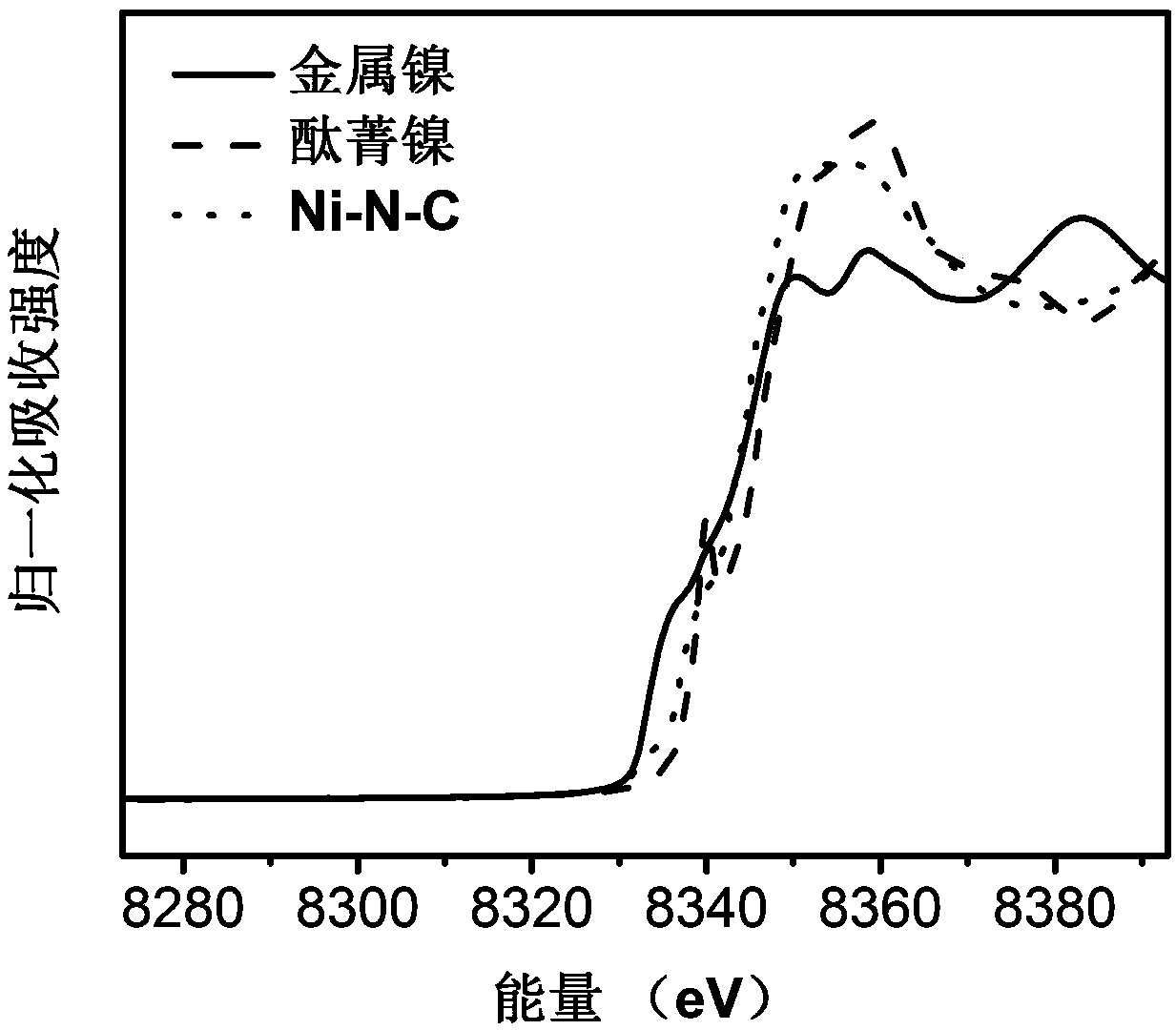
Ni-N-C catalyst for electroreduction reaction of carbon dioxide and preparation and application thereof
ActiveCN109652821AImprove efficiencyIncrease current densityElectrolytic organic productionElectrodesFaraday efficiencyZinc nitrate
The invention discloses a preparation method of a Ni-N-C catalyst for a carbon dioxide electroreduction reaction. The method comprises the following steps: dissolving zinc nitrate and nickel nitrate in methanol according to a certain ratio, adding a certain amount of 2-methylimidazole methanol solution while stirring, and stirring the mixture at room temperature for 12-36 hours; centrifuging and washing with methanol for 3 times, drying under vacuum to obtain a solid powder; taking a certain amount (0.1-0.8g) of the solid powder in a tube furnace, heating to 600-1000 DEG C at a certain flow velocity and under the protection of inert gas, maintaining for 1 to 12 hours, and cooling to room temperature to obtain the Ni-N-C catalyst. The catalyst body is amorphous carbon with N and Ni uniformly doped in a carbon skeleton, is applied to carbon dioxide electroreduction, and has high CO Faraday efficiency and current density.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Thickness-controllable bismuth nanosheet and preparation method and application of alloy
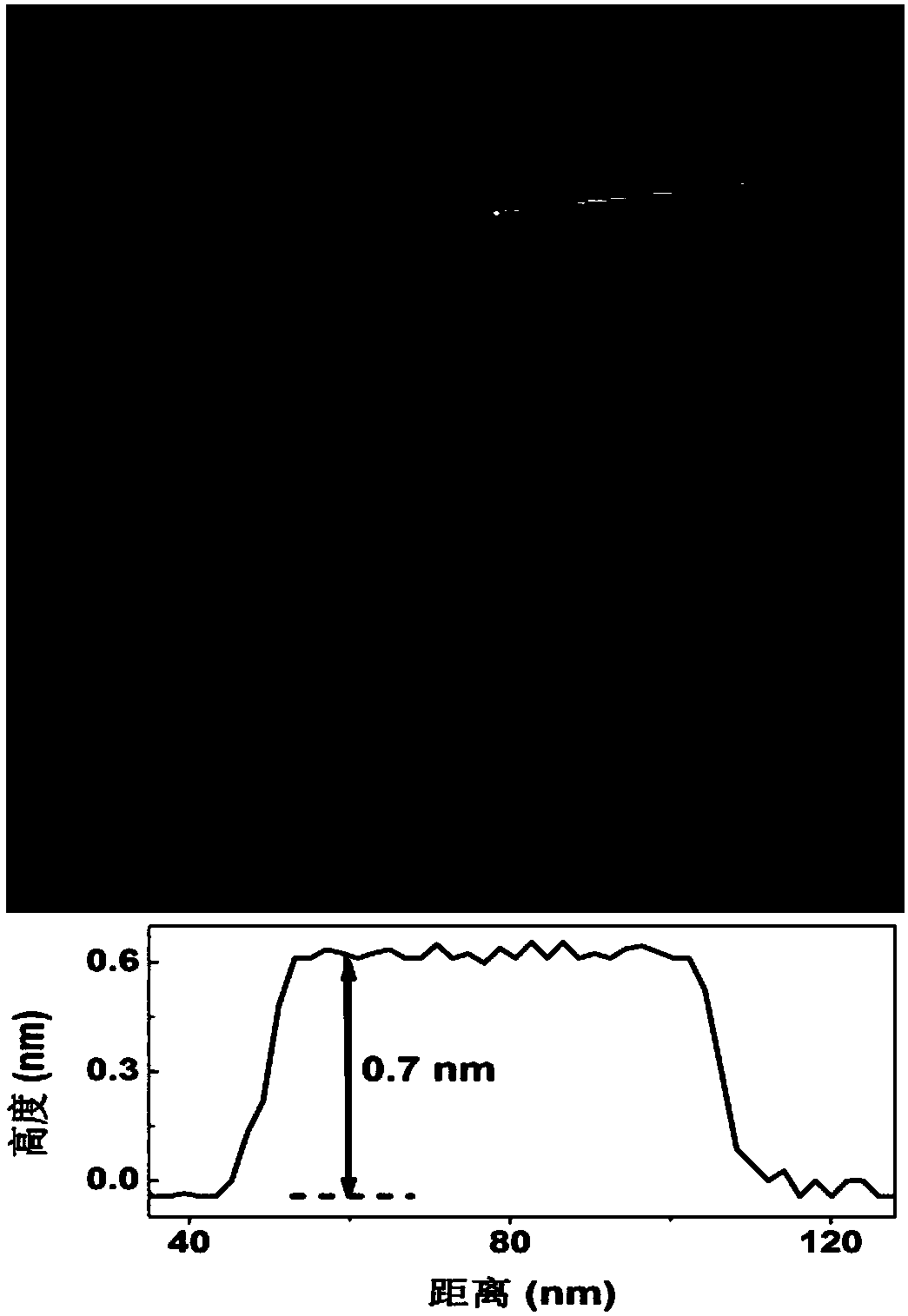
ActiveCN108480656AStrong reductionAdjustable thicknessMaterial nanotechnologyElectrolysis componentsFaraday efficiencyAlloy
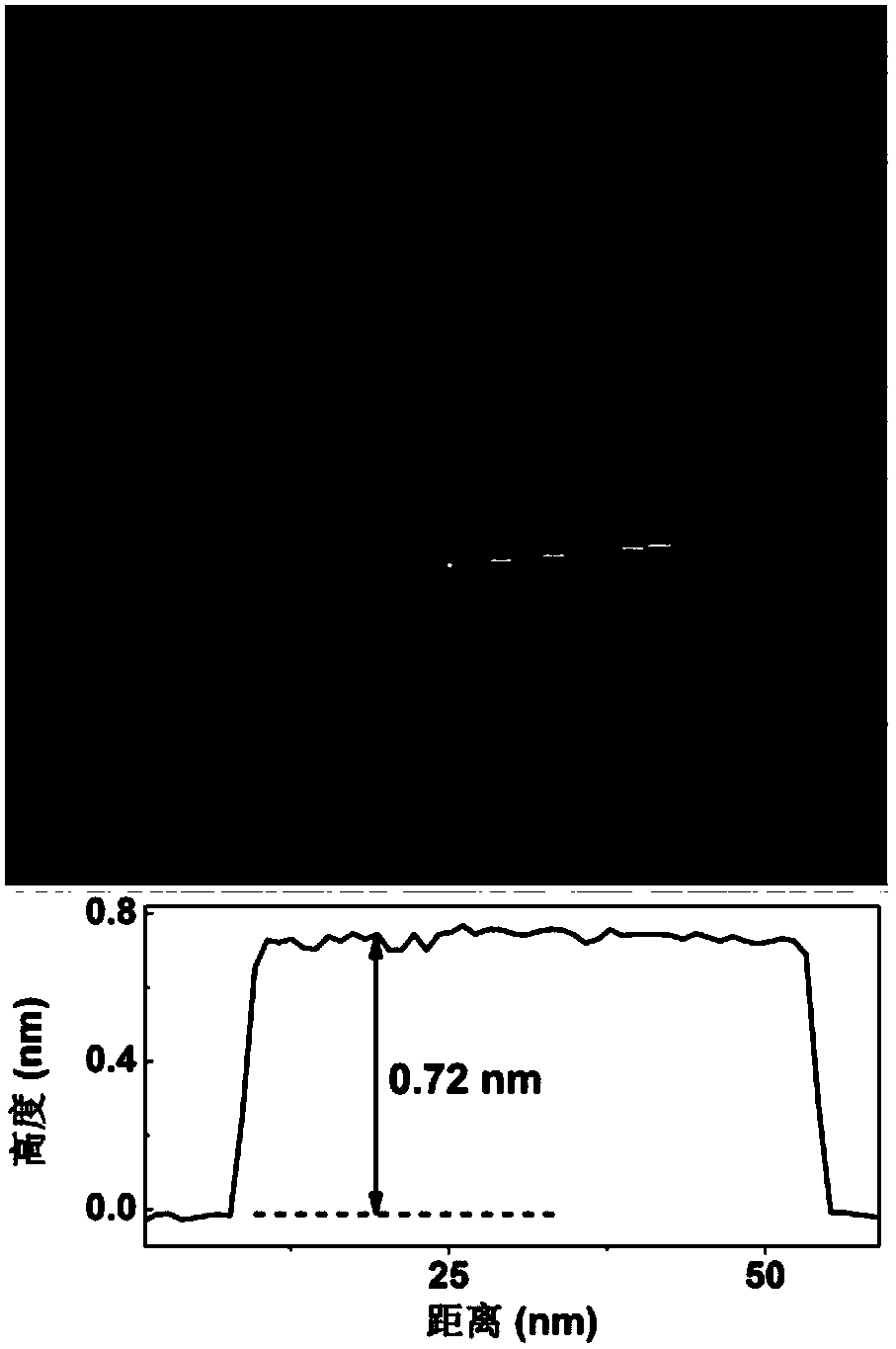
The invention relates to a thickness-controllable bismuth nanosheet, and a preparation method and application of an alloy, and aims at solving the technical problems that when a traditional metal catalyst is used for converting carbon dioxide into formic acid, the efficiency is low, the overpotential is high, the hydrogen evolution potential is relatively positive, and the stability is poor. The bismuth nanosheet with the thickness of only 0.7nm monatomic layer is obtained for the first time by using salts of bismuth as raw materials, ethylene glycol monoethyl ether as a solvent and a solutionwith strong reducibility such as NaBH4 or LiBH4 as a reducing agent through an aqueous solution reduction method under the protection of an inert gas, and the thickness of the bismuth nanosheet is adjustable. The thickness controlled bismuth nanosheet prepared by the method shows excellent CO2 catalytic reduction performance; the Faradic efficiency of catalyzing CO2 to generate formic acid by using the bismuth nanosheet in the case of overpotential being 330mV can reach 98%; the initial overpotential of the bismuth nanosheet is as low as 80mV; the stability of the bismuth nanosheet is as longas 75h; and even the bismuth nanosheet is treated for 4h at the temperature of 300 DEG C, the thickness and catalytic performance of the bismuth nanosheet hardly change, thus further the fact that the bismuth nanosheet has superhigh stability is verified.
Owner:CHANGCHUN INST OF APPLIED CHEMISTRY - CHINESE ACAD OF SCI
Ion-Conducting Membranes
An anion-conducting polymeric membrane comprises a terpolymer of styrene, vinylbenzyl-Rs and vinylbenzyl-Rx. Rs is a positively charged cyclic amine group. Rx is at least one constituent selected from the group consisting Cl, OH and a reaction product between an OH or Cl and a species other than a simple amine or a cyclic amine. The total weight of the vinylbenzyl-Rx groups is greater than 0.3% of the total weight of the membrane. In a preferred embodiment, the membrane is a Helper Membrane that increases the faradaic efficiency of an electrochemical cell into which the membrane is incorporated, and also allows product formation at lower voltages than in cells without the Helper Membrane.
Owner:DIOXIDE MATERIALS
Gas diffusion electrode, preparation method thereof and application thereof in electrochemical reduction of carbon dioxide
ActiveCN108823596AIncrease the catalytic activity of electrochemical reductionImprove Faraday efficiencyElectrolytic organic productionMetal/metal-oxides/metal-hydroxide catalystsFaraday efficiencyElectrochemical reduction of carbon dioxide
The invention relates to a gas diffusion electrode, a preparation method of the gas diffusion electrode, and the application of the gas diffusion electrode in electrochemical reduction of carbon dioxide. The gas diffusion electrode comprises a gas diffusion electrode body and carbon dioxide electrochemical reduction catalysts loaded on the gas diffusion electrode body. Nano copper oxide supportedon graphite phase carbon nitride serves as the carbon dioxide electrochemical reduction catalysts and has two crystal forms of tenorite and cuprit. The gas diffusion electrode can improve the Faradayefficiency of carbon dioxide electrochemical reduction products such as methane, ethylene and other hydrocarbons, and also can effectively inhibit hydrogen evolution reaction.
Owner:ZHEJIANG UNIV
Method for preparing 2,5-furandicarboxylic acid through electrocatalytic oxidation by using nickel-vanadium phosphide catalyst
ActiveCN109837555AWide range of resources and cheapLow costPhysical/chemical process catalystsElectrolytic organic productionFaraday efficiencyHydroxymethylfurfural
The invention relates to a method for preparing 2,5-furandicarboxylic acid by electrocatalytic oxidation of a nickel-vanadium phosphide catalyst. A reaction is carried out in an H-shaped electrolyticcell; in an anode chamber, the nickel-vanadium phosphide catalyst servers as a working electrode, and 5-hydroxymethylfurfural, serving as a reaction substrate, is dissolved in an alkaline solution toform an anode solution; in a cathode chamber, a platinum sheet is used as a counter electrode, and an alkaline solution is used as a cathode solution, so that an electrocatalytic oxidation reaction iscarried out for 0.5-3 h under the conditions that the temperature is 20-60 DEG C, the current is 5-50 mA and the cell voltage is 1-20 V; and after the reaction is finished, post-treatment is carriedout to obtain the 2,5-furandicarboxylic acid. The electrocatalytic oxidation reaction process of the method is mild in condition, so that the method is green and pollution-free, the raw material conversion rate is high, the FDCA selectivity is good, and the Faraday efficiency is high. Compared with precious metal catalysts commonly adopted in the prior art, the transition metal nickel-vanadium phosphide catalyst used in the method is low in cost, so that consumption of rare precious metal is avoided.
Owner:ZHEJIANG UNIV OF TECH
CuZn double-monatomic electrochemical catalysis CO2 reduction material and preparation method thereof
ActiveCN111841601AGood dispersionThe experimental method is simplePhysical/chemical process catalystsElectrolytic organic productionFaraday efficiencyPhysical chemistry
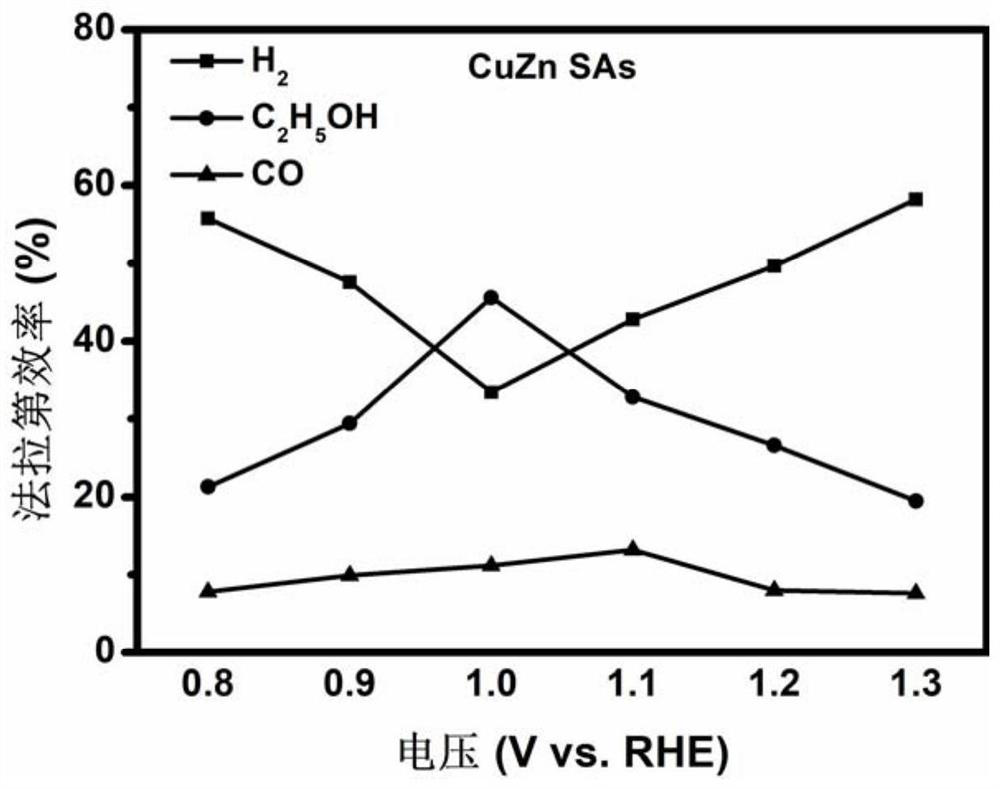
The invention discloses a CuZn double-monatomic electrochemical catalysis CO2 reduction material and a preparation method thereof, and belongs to the field of composite material preparation. The electrochemical catalysis CO2 reduction material is composed of a reaction active matter and a carrier, CuZn double-monatoms are used as the reaction active matter, and nitrogen-doped carbon nanosheets areused as the carrier; the metals Cu and Zn exist in the form of single atoms and have the maximum atom utilization efficiency, so that the electrocatalytic material has relatively excellent catalyticactivity and selectivity, and the Faraday efficiency of a product ethanol is close to 50% within a relatively wide voltage test range (-0.8 to -1.3 V vs.RHE).
Owner:JIANGNAN UNIV
Method for preparing furan-2,5-dicarboxylicacid from 5-hydroxymethylfurfural through electrochemical oxidation
ActiveCN110106514AEnhanced electrochemical oxidationImprove Faraday efficiencyElectrolytic organic productionMetal/metal-oxides/metal-hydroxide catalystsFuranFaraday efficiency
The invention relates to a method for furan-2,5-dicarboxylicacid from 5-hydroxymethylfurfural through electrochemical oxidation. The method includes the following steps that an H-type double-electrochemical-cell reactor is adopted and divided into a cathode chamber and an anode chamber through a proton exchange membrane for separation; a three-electrode system is adopted, a catalyst supported electrode serves as a working electrode, a platinum electrode serves as an auxiliary electrode, and a silver / silver chloride electrode serves as a reference electrode. The working electrode includes a working electrode body and a 5-hydroxymethylfurfural electrochemical oxidation catalyst supported on the working electrode body, wherein the 5-hydroxymethylfurfural electrochemical oxidation catalyst isthe activated carbon supported oxidized-state or reduced-state copper-nickel bimetallic catalyst. The method can improve the yield and Faradic efficiency of the product, namely, furan-2,5-dicarboxylicacid.
Owner:ZHEJIANG UNIV
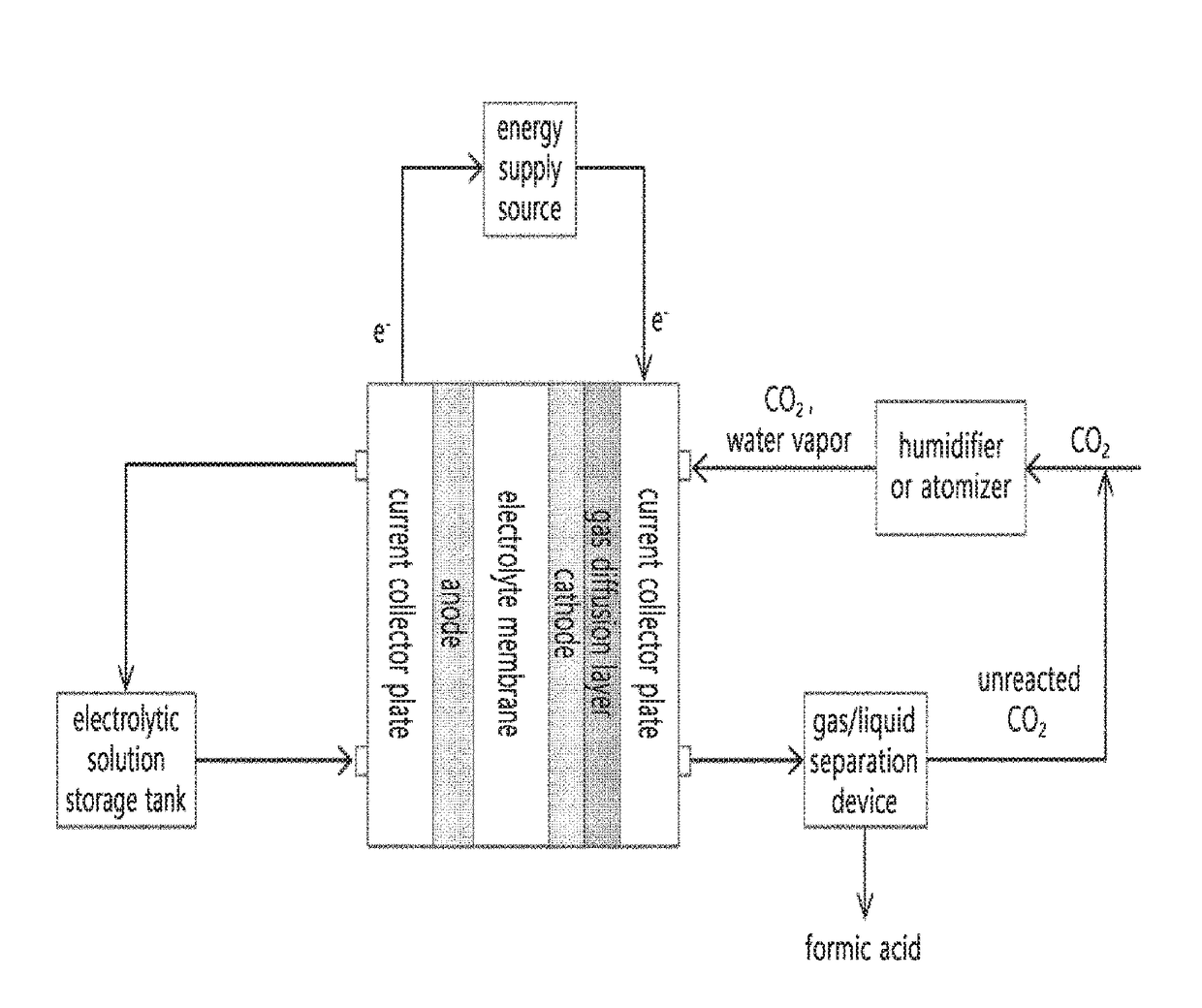
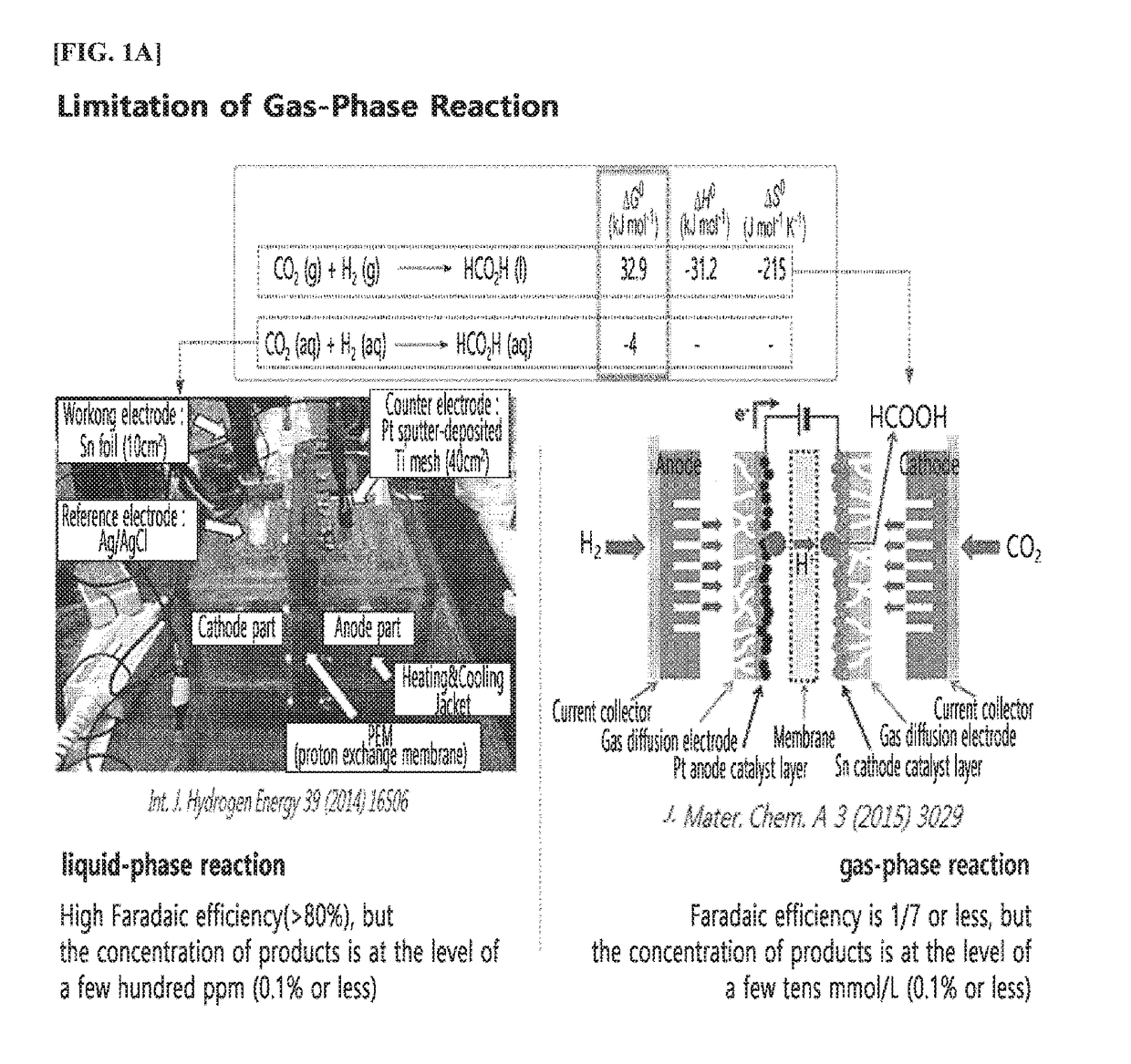
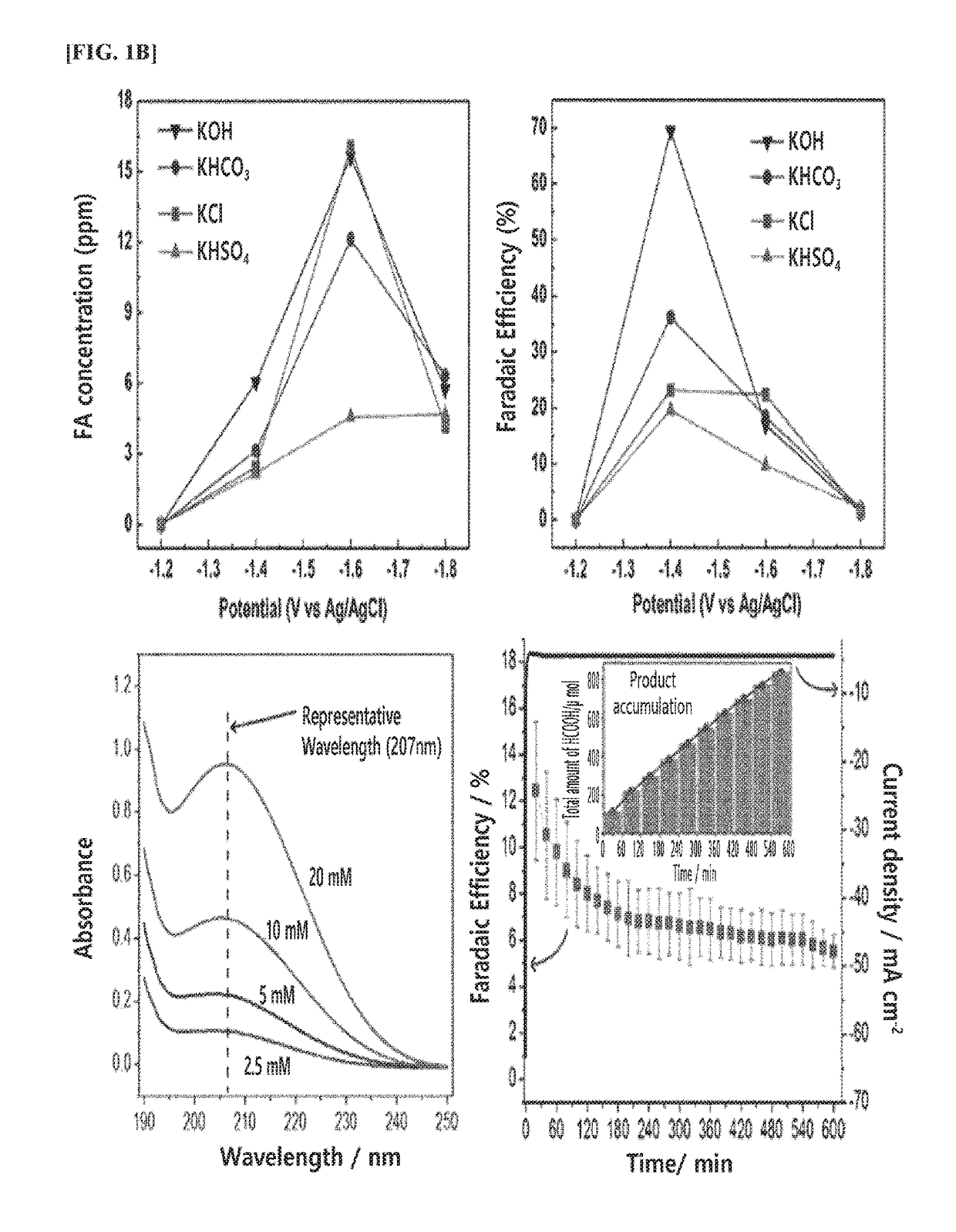
Method and apparatus for preparing reduction product of carbon dioxide by electrochemically reducing carbon dioxide
ActiveUS20180202056A1Improve Faraday efficiencyReducing the carbon dioxide gasOrganic compound preparationDispersed particle separationHigh concentrationHydrogen
The present invention relates to a method and an apparatus of preparing a reduction product of carbon dioxide by electrochemically reducing carbon dioxide.The present invention can prepare, in an energy-efficient manner, a reduction product of high-concentration carbon dioxide with high Faraday efficiency as in a liquid reduction reaction by producing the reduction product of carbon dioxide by supplying water or an electrolytic solution to an anode region; supplying humidified carbon dioxide gas having a second temperature higher than a first temperature to a cathode region within an electrochemical cell having the first temperature so as to supply the carbon dioxide gas which has been humidified to be in a condition where the relative humidity is greater than 100%, while applying a voltage between the anode region and the cathode region so as to generate hydrogen ions (H+) in the anode region; and transporting the hydrogen ions to the cathode region through the electrolyte membrane, thereby electrochemically reducing the carbon dioxide gas.
Owner:KOREA INST OF ENERGY RES
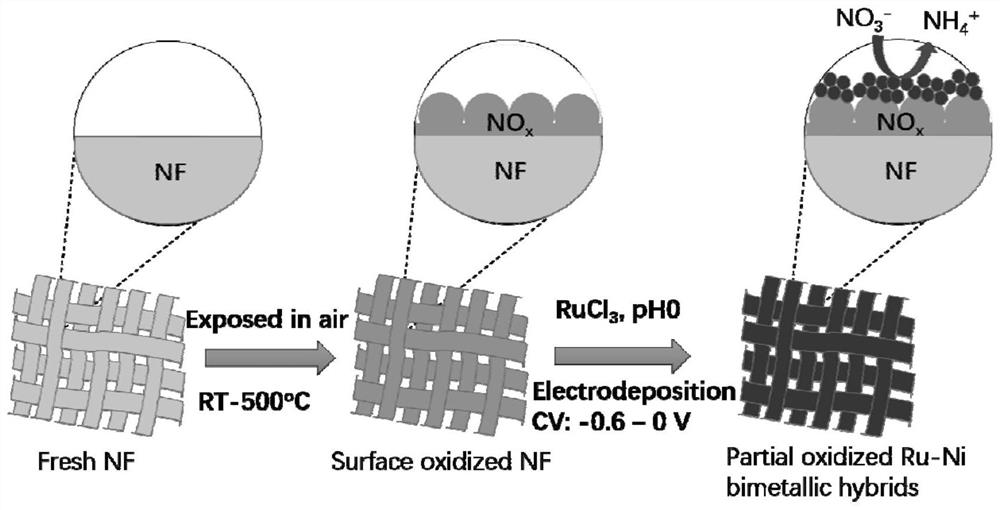
Catalyst for electrocatalytic reduction of nitrate as well as preparation method and application thereof
PendingCN112237927AEnhanced catalytic performance for reductionLow load RuWater contaminantsCatalyst activation/preparationPtru catalystNitrate
The invention relates to the technical field of catalyst preparation, in particular to a preparation method of a catalyst for electrocatalytic reduction of nitrate. The method comprises the followingsteps: by using foamed nickel as a substrate, forming a nickel oxide layer on the surface of the foamed nickel to obtain a compound; and depositing ruthenium nanoparticles on the compound by adoptinga ruthenium trichloride solution and adopting an electrochemical cyclic voltammetry to obtain the NiRu composite catalyst. According to the preparation method of the catalyst for electrocatalytic reduction of nitrate, the preparation method is simple in process, the catalyst load Ru amount is low, and the catalyst cost is low; the invention also provides an application of the catalyst for electrocatalytic reduction of nitrate. The catalyst has higher current density, higher current Faraday efficiency and higher nitrate elimination rate of synthetic ammonia in a wide voltage range.
Owner:DONGGUAN UNIV OF TECH
Preparation and application of carbon dioxide electrochemical reduction catalyst
InactiveCN112791739AReduce reunionActs as an ion bufferCatalyst activation/preparationElectrodesPtru catalystFaraday efficiency
The invention relates to preparation and application of a carbon dioxide electrochemical reduction catalyst. The preparation method of the catalyst comprises the following steps of by taking carbon spheres rich in microporous structures as a precursor, limiting adsorbed transition metal ions into a pore structure of the carbon spheres by utilizing the microporous structures of the carbon spheres through a liquid-phase impregnation process, and introducing a nitrogen source into later-stage high-temperature calcination carbonization treatment, generating a nitrogen-rich sheet structure at a high temperature to further anchor the transition metal monatomic. Therefore, the high-dispersion and high-load monatomic catalyst is prepared. The preparation method of the catalyst provided by the invention is simple / easy to control, has excellent Faraday efficiency, catalytic activity and stability for preparing CO through CO2 electrochemical reduction, and is expected to realize large-scale commercial production.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Electrocatalytic material for converting nitrogen gas into ammonia gas
InactiveCN109647464AProcess environmental protectionSimple processPhysical/chemical process catalystsElectrodesFaraday efficiencyAmmonia production
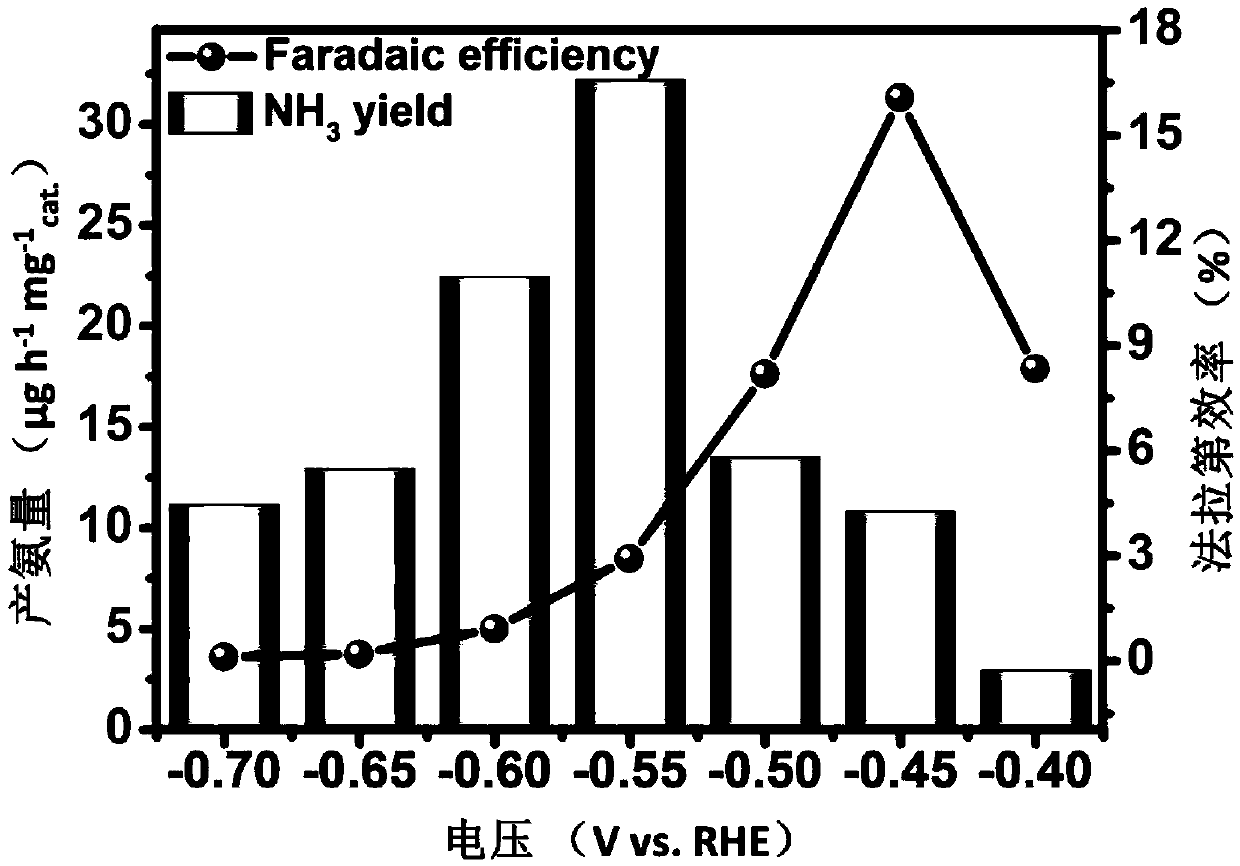
The invention belongs to the field of preparation of nano materials and nitrogen reduction, and relates to an electrocatalytic material for converting nitrogen gas into ammonia gas. Pure Ti3C2Tx MXeneis prepared by using a Ti3AlC2 precursor at first and then improved through a solvothermal mode, and finally, the use method and catalytic effect of pure Ti3C2Tx MXene are verified; it is found thatthe ammonia production amount of a Ti3C2Tx / TiO2 nano composite can reach 32.17 micrograms h<-1>mg<-1>cat. at -0.55V, the Faraday efficiency of the composite reaches 16.07% at -0.45V,but the ammonia production amount of pure Ti3C2Tx MXene only reaches 22.19 micrograms h<-1>mg<-1>cat. at -0.55V, the Faraday efficiency of the composite reaches 7.09% at -0.45V, the ammonia production amount and Faraday efficiency of the Ti3C2Tx / TiO2 nano composite are 11 times and 69 times those of TiO2 nano particles respectively, and the catalytic efficiency of the Ti3C2Tx / TiO2 nano composite is higher than that of most of the current electrocatalysts; it is shown that the Ti3C2Tx / TiO2 nano composite is a very good nitrogen reduction material, can be applied to the field of nitrogen reduction, is simple andeasy to operate, the raw materials are easy to obtain, and the preparation cost is low; the preparation efficiency is high, the quality of the prepared product is high, the stability is high, the application environment is good, and the market prospect is broad.
Owner:QINGDAO UNIV
Metal-nitrogen-carbon catalyst for carbon dioxide electroreduction and preparation method of metal-nitrogen-carbon catalyst
PendingCN111686780AImprove performanceGood dispersionCatalyst activation/preparationElectrodesPtru catalystFaraday efficiency
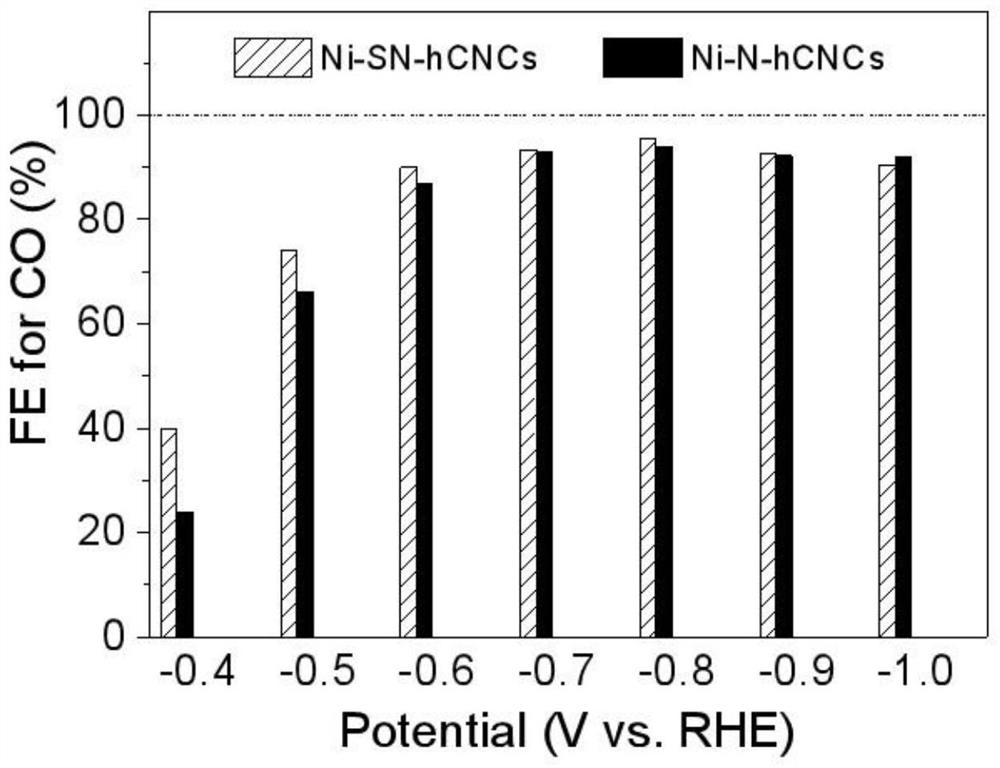
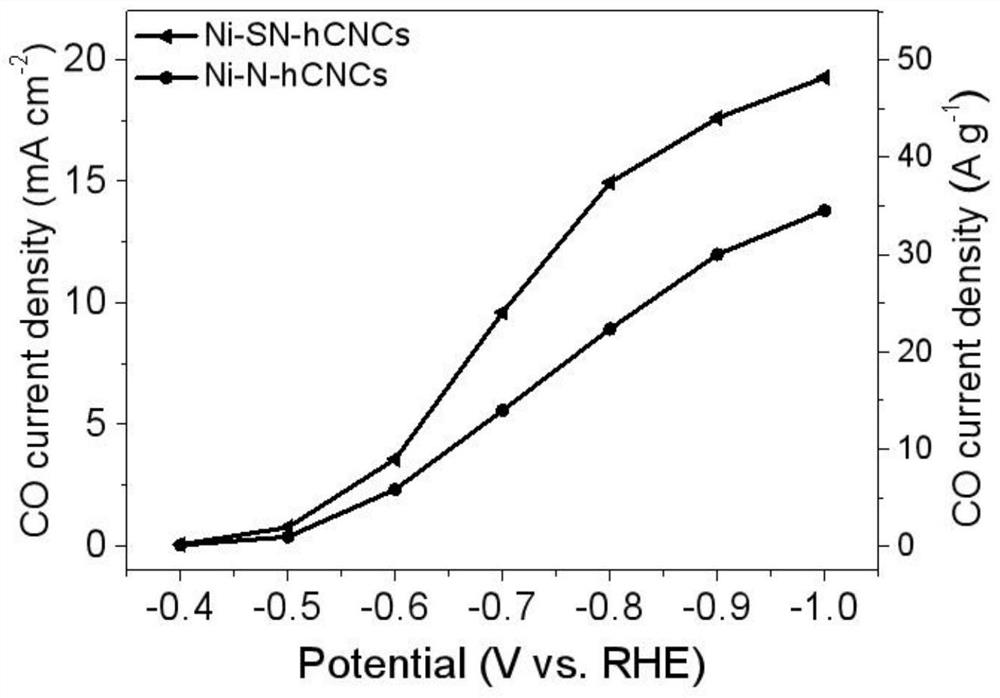
The invention provides a metal-nitrogen-carbon catalyst for carbon dioxide electroreduction. The metal-nitrogen-carbon catalyst comprises a carrier and an active component. Metal-nitrogen-carbon unitpoints are loaded on the surface of carbon-based nanocages, and the dispersity and the loading capacity of the unit points are greatly improved by utilizing the three-dimensional hierarchical structure and the high specific surface area of the nanocages. The nickel-nitrogen-carbon single-site catalyst is used for carbon dioxide electroreduction, and the Faraday efficiency of a carbon monoxide product reaches 87-94% at a wide voltage window of -0.6 V to -1.0 V; through sulfur doping, the specific current density of carbon monoxide is increased from 22.3 Ag<-1> to 37.5 Ag<-1>, and the Faraday efficiency of carbon monoxide reaches 90-95% in the same voltage window.
Owner:NANJING UNIV
Carbon dioxide electrochemical reduction catalyst and preparation method thereof and catalyst loaded gas diffusion electrode
InactiveCN108654623AMany active sitesReduce overpotentialCatalyst activation/preparationElectrolytic organic productionNano catalystFaraday efficiency
The invention relates to the technical field of electrochemical reduction catalysts, in particular to a carbon dioxide electrochemical reduction catalyst and a preparation method thereof and a catalyst loaded gas diffusion electrode. The carbon dioxide electrochemical reduction catalyst is of a nano-catalyst and is specifically a nickel-copper lamellar bimetallic oxide with a composite lamellar structure of nickel-copper oxide and copper oxide, or nickel-tin lamellar bimetallic oxide with a composite lamellar structure of nickel oxide and tin oxide. The nano-catalyst with the structure is highin catalytic activity, overpotential in electrochemical reduction process is greatly reduced, and meanwhile, hydrogen evolution reaction in the process of carbon dioxide reduction is effectively restrained. The invention further provides the preparation method of the catalyst and the catalyst loaded gas diffusion electrode, the problem about excessive low reduction current can be solved, and theFaradic efficiency in the electrochemical reduction of carbon dioxide is effectively improved.
Owner:CHANGCHUN INST OF APPLIED CHEMISTRY - CHINESE ACAD OF SCI
Method for synthesizing olefin by electro-catalyzing semi-hydrogenated gas-phase alkyne
PendingCN112301369AHigh selectivityGood choiceElectrolytic organic productionElectrodesChemical industryPtru catalyst
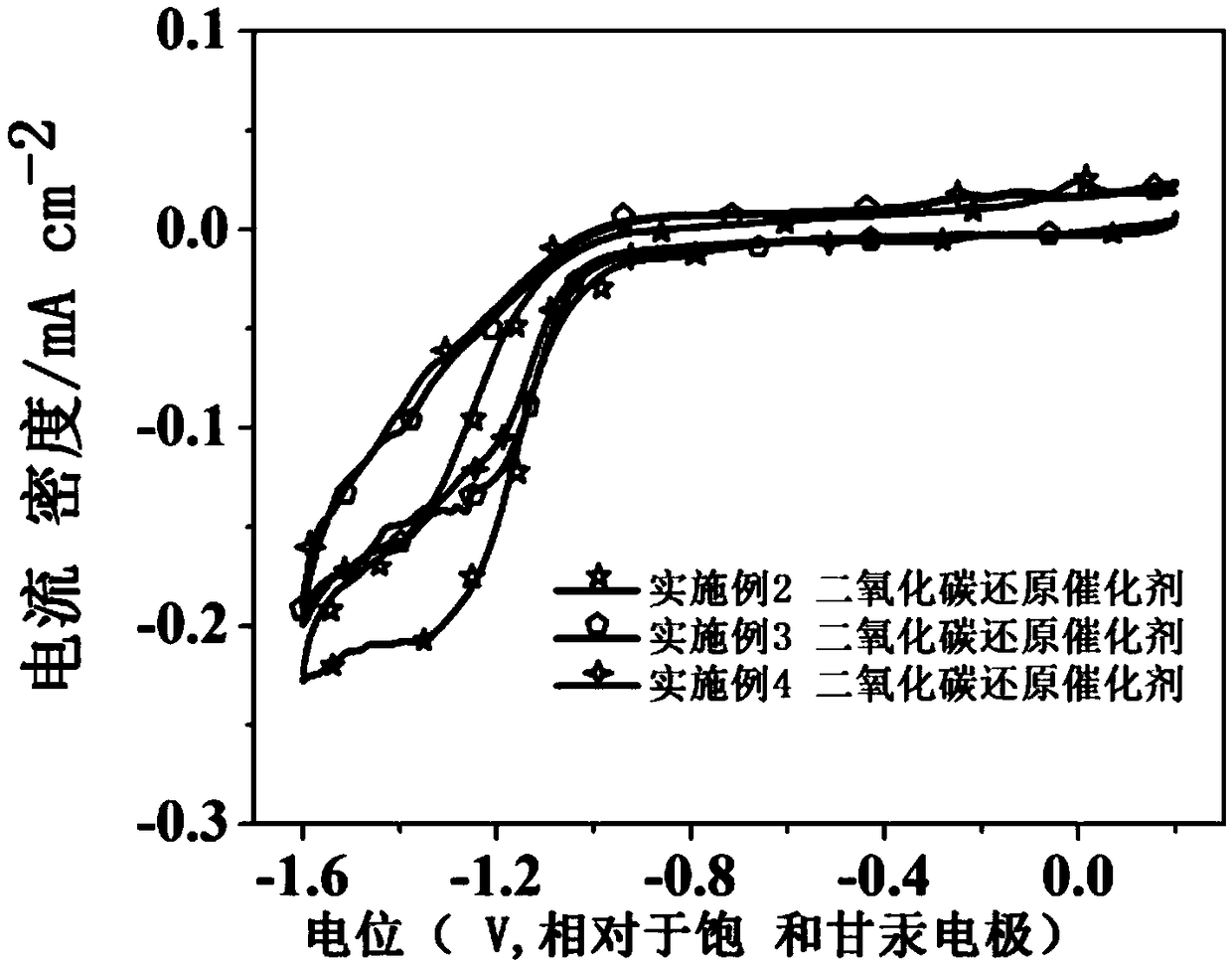
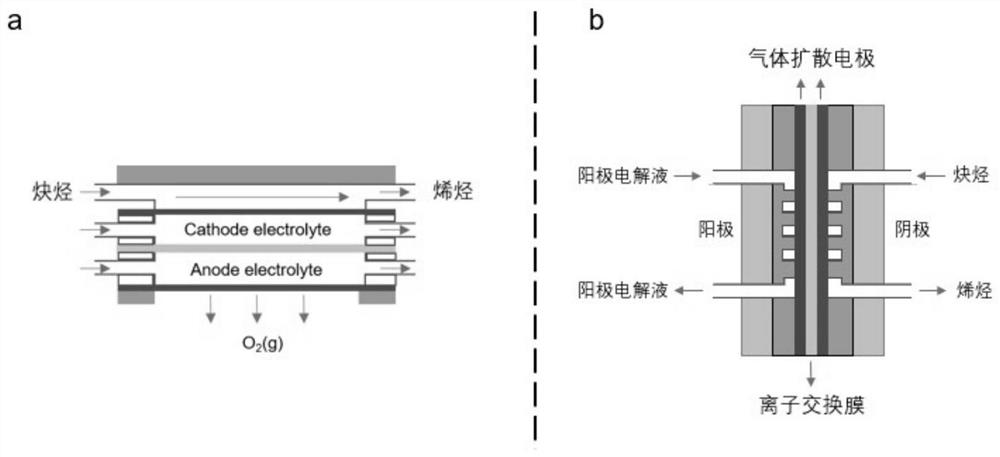
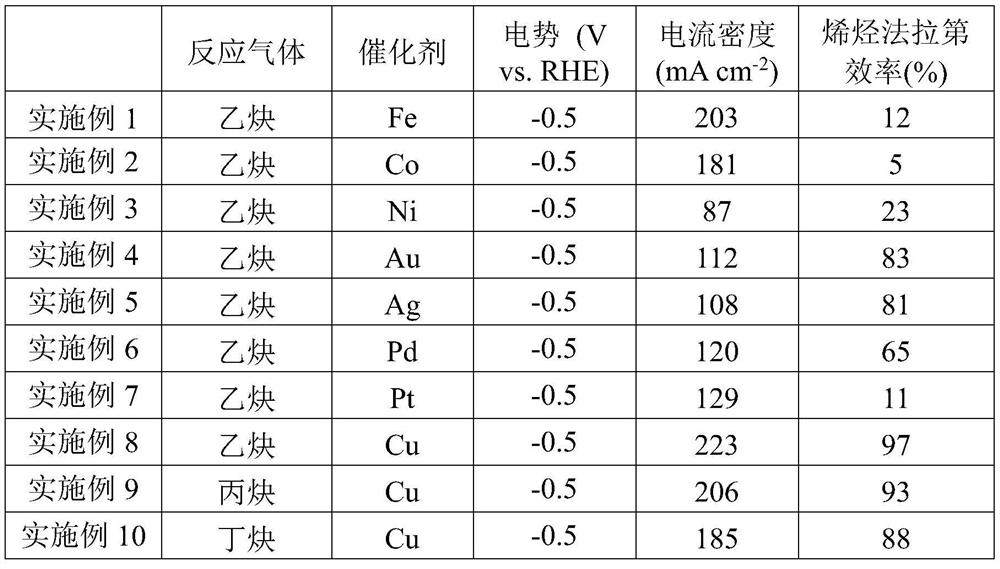
The invention relates to a method for synthesizing olefin by electro-catalyzing semi-hydrogenated gas-phase alkyne. The method comprises the following steps of: spraying a catalyst onto a gas diffusion layer substrate (comprising conductive carbon paper, metal and the like) by using a gas diffusion electrode, isolating a cathode from an anode by using an ion exchange membrane, and adopting a three-electrode or two-electrode system constant-voltage method to carry out electrochemical performance test, wherein the reaction gas is high-purity alkyne. According to the invention, the experimental results show that the method can be used for efficiently and selectively reducing the gas-phase alkyne into the corresponding olefin; compared with an H-type electrolytic tank, after the gas diffusionelectrode is used, the reaction current density is multiplied, the reaction voltage and the reaction current can reach 1 Acm<-2> or above by regulating and controlling the reaction voltage, and the Faraday efficiency of a target olefin product is remarkably improved and reaches 95% or above; and compared with the traditional thermal catalysis technology, the method can selectively reduce the gas-phase alkyne into olefin at normal temperature and normal pressure without hydrogen consumption, can greatly reduce the energy consumption in the process, better meets the requirements of green chemical industry, and has great strategic significance.
Owner:NORTHWESTERN POLYTECHNICAL UNIV
Electrode for electrochemical reduction of carbon dioxide, as well as preparation method and application of electrode
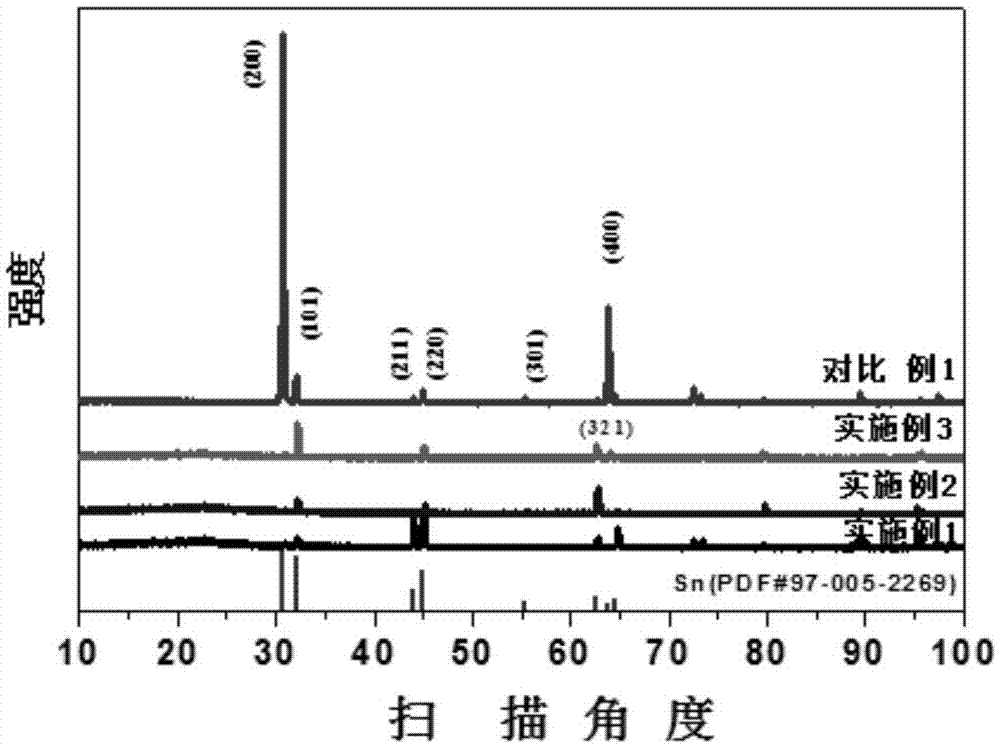
The invention discloses an electrode for electrochemical reduction of carbon dioxide, as well as preparation and application of the electrode. The electrode comprises a basal layer and a Sn catalyst nanolayer attached to the basal layer, wherein the carrying capacity of a Sn catalyst is 1mg / cm<-2> to 6mg / cm<-2>; and Sn in the electrode is composed of one, two or more of 1-500nm nanowire, nanorod and nanofiber. According to the invention, the Sn nanorod with rich edge active sites grows on a base in situ, so that the specific area and active area of the electrode are remarkably increased, and the Faraday efficiency of electrochemical reduction of carbon dioxide into formic acid by the electrode is improved.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Iron-based non-noble metal catalyst for electrocatalytic synthetic ammonia and preparation method thereof
The invention discloses an iron-based non-noble metal catalyst for electrocatalytic synthetic ammonia and a preparation method thereof. The preparation method is characterized in that bivalent iron ions in the bivalent iron salt solution are reduced and deposited on the surface of a conductive carrier by adopting an electrochemical deposition method, and the bivalent iron salt solution contains bivalent iron salt, a reducing agent, o-sulfonyl imide and sodium dodecyl sulfate. According to the preparation method, the catalyst is carried out electro-catalysis to synthesize ammonia, so that the faraday efficiency of the electrocatalysis synthetic ammonia process can be obviously improved.
Owner:SHANDONG NORMAL UNIV
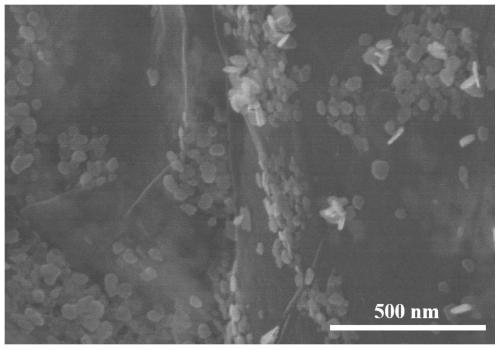
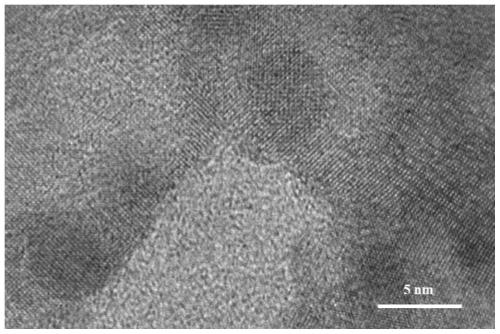
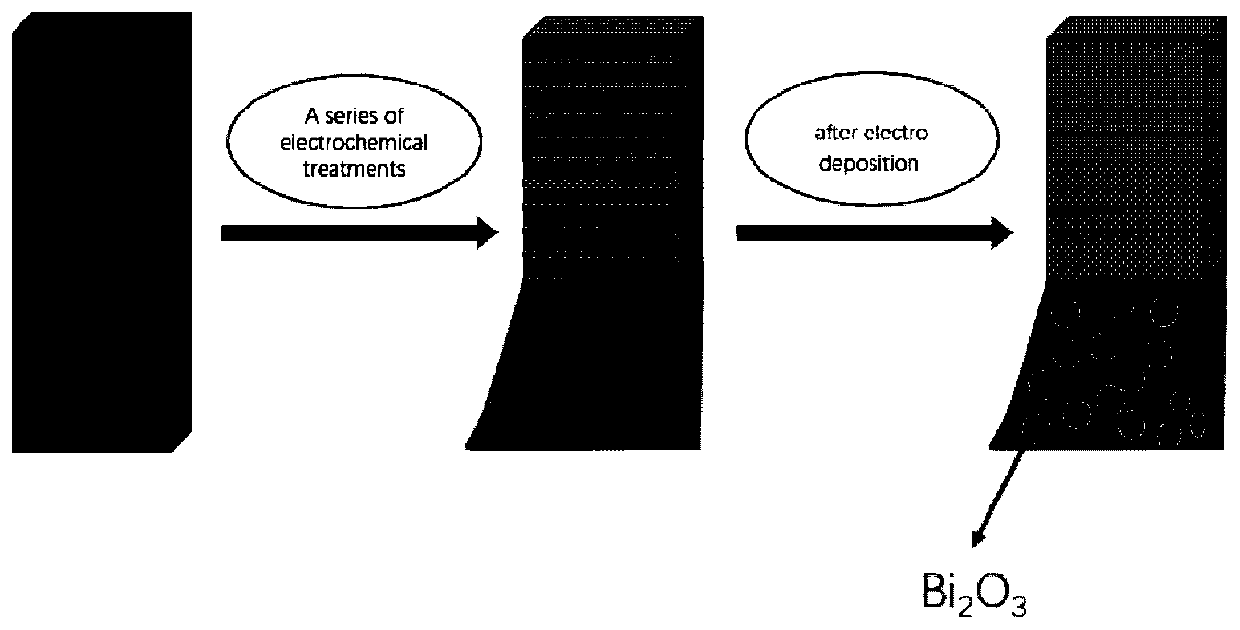
Bismuth-based self-supporting electrocatalyst, preparation method thereof and application of bismuth-based self-supporting electrocatalyst in nitrogen reduction ammonia production
PendingCN111359603AHigh catalytic efficiencyIncrease layer spacingElectrolytic inorganic material coatingCatalyst activation/preparationElectrolytic agentPtru catalyst
The invention belongs to the technical field of catalysts, and particularly relates to a preparation method of a bismuth-based self-supporting electrocatalyst and an application of the bismuth-based self-supporting electrocatalyst in electrocatalytic nitrogen reduction ammonia production. Bi2O3 catalyst nanoparticles are immobilized on a carbon material substrate, treated by an electrochemical method, by the electrochemical method to obtain the bismuth-based self-supporting electrocatalyst. Electrodeposition is carried out by taking carbon material treated by the electrochemical method as a working electrode, a platinum sheet electrode as a counter electrode and a reference electrode and an ethylene glycol solution of BiCl3 as an electrolyte, and the obtained carbon material substrate immobilized with the Bi2O3 catalyst nanoparticles is taken out, cleaned and dried to obtain the bismuth-based self-supporting electrocatalyst. The bismuth-based self-supporting electrocatalytic material stable in structure, good in conductivity and large in specific surface area is obtained. The bismuth-based self-supporting electrocatalyst has the advantages of simple preparation method, good repeatability, no need of a binder, adjustable immobilization amount, ideal ammonia production efficiency and Faraday efficiency of electrocatalytic nitrogen reduction, and wide application prospect in the fields of electrocatalytic nitrogen reduction ammonia production and the like.
Owner:LIAONING UNIVERSITY
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com