Electrode for electrochemical reduction of CO2 and preparation of formic acid and preparation method and application thereof
A CO2, electrochemical technology, applied in the field of electrochemistry, can solve the problem of less catalytic active points on the surface of the metal sheet electrode, and achieve the effects of increased current density, stable structure, and high specific surface area.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
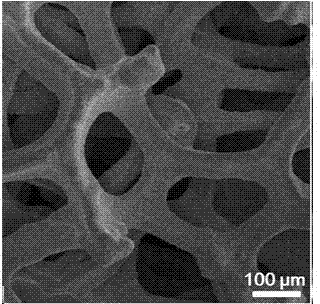
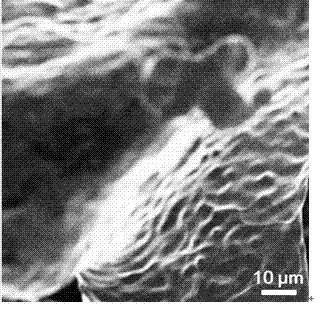
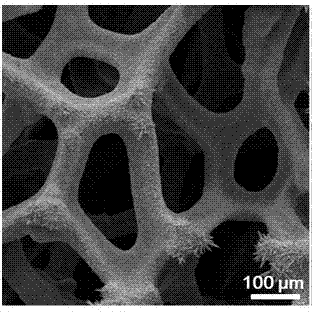
[0031] Example 1: The electroplating solution is an aqueous electroplating solution, sodium citrate is added to deionized water to make 0.009 mol L -1 aqueous solution, and stirred at room temperature to dissolve it, and then added 0.003 mol L -1 of stannous chloride, stirred for 10 h. The copper foam was then placed in this electroplating solution at 1 mA cm -2 After constant current electroplating under high temperature for 15 min, it was ultrasonically rinsed with ethanol and deionized water, and dried to obtain a Sn / Cu electrode. figure 1 , 2 and image 3 , 4 SEM images of copper foam and Sn / Cu electrodes, respectively, can confirm that tin particles have been successfully loaded on the surface of copper foam (see image 3 , 4 ). At the same time by Figure 5 It can be seen from the linear voltammogram shown that the reduction current density of the Sn / Cu electrode is significantly higher than that of the Sn sheet electrode, which indicates that the Sn / Cu electrode...
Embodiment 2
[0032] Embodiment 2: The electroplating solution is an aqueous electroplating solution, and sodium citrate is added into deionized water to make 0.02 mol L -1 Aqueous solution, and stirred at room temperature to dissolve, then add 0.005 mol L -1 indium sulfate, stirred for 12 h. The copper foam was then placed in the electroplating solution at 3 mA cm -2 After constant current electroplating under high temperature for 30 min, it was ultrasonically rinsed with ethanol and deionized water, and dried to obtain an In / Cu electrode. With the In / Cu electrode as the working electrode (cathode), the graphite electrode as the auxiliary electrode (anode), and the silver-silver chloride electrode as the reference electrode, the electrolyte solution is filled into the electrolytic cell, and CO 2 to saturation, and then continue to feed CO 2 Electrolytic reduction is carried out to produce formic acid under optimal conditions, and a high Faradaic efficiency of more than 85% can be obtain...
Embodiment 3
[0033] Example 3: The electroplating solution is an aqueous electroplating solution, and sodium citrate is added to deionized water to make 0.06 mol L -1 Aqueous solution, and stirred at room temperature to dissolve, then add 0.015 mol L -1 bismuth nitrate, stirred for 16 h. Then place copper foam in this electroplating solution at 5 mA·cm -2 After constant current electroplating under high temperature for 60 min, it was ultrasonically rinsed with ethanol and deionized water, and dried to prepare a Bi / Cu electrode. With Bi / Cu electrode as working electrode (cathode), IrO 2 ·RuO 2 The coated titanium electrode is the auxiliary electrode (anode), the silver silver chloride electrode is the reference electrode, the electrolyte solution is filled into the electrolytic cell, and CO 2 to saturation, and then continue to feed CO 2 Electrolytic reduction is carried out to produce formic acid under optimal conditions, and a high Faradaic efficiency of more than 85% can be obtained...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com