High-performance ultrathin nitride electro-catalyst with functions of producing hydrogen and oxygen by means of electrochemically totally decomposing water, method for synthesizing high-performance ultrathin nitride electro-catalyst and application thereof
A technology of nitride electricity and synthesis method, which is applied in the direction of physical/chemical process catalysts, chemical instruments and methods, chemical/physical processes, etc., to achieve the effects of low cost, large specific surface area, good conductivity and stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
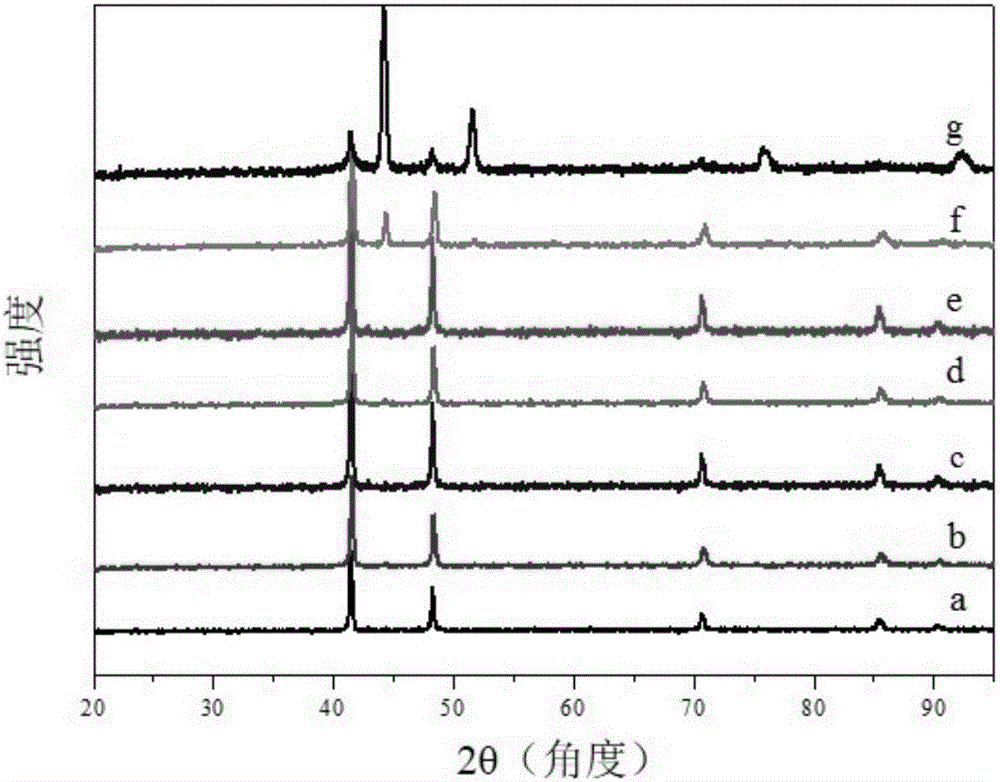
[0048] Synthesis of comparison samples:
[0049] Synthesis of NiFe-MMO
[0050] (1) Synthesis of NiFe hydrotalcite (NiFe-LDH) precursor: 5 mL of n-butanol and 8 mL of oleylamine were placed in a reactor, stirred at high speed to obtain a microemulsion environment, and Ni 2+ , Fe 3+ Add it into the reactor in a certain proportion, mix it evenly, and then hydrothermally crystallize it in a high airtight system for 6-48h. After the reaction, the product was centrifuged and washed with ethanol-water mixed solution, and dried to obtain NiFe-LDH.
[0051] (2) High temperature oxidation: put NiFe-LDH in a muffle furnace, program the temperature to 400-700°C, and keep the high temperature for 5-10h, the heating rate is 5°C / min-10°C / min, and the dark brown oxidation product NiFe is obtained -MMOs.
[0052] Ni 3 Synthesis of N
[0053] Put NiO in an atmosphere tube electric furnace, program the temperature to 300-600°C in an ammonia atmosphere, and keep the high temperature for 3-...
Embodiment 2
[0055] FeNi with ultrathin nanosheet structure 3 The preparation method of N electrocatalyst comprises:
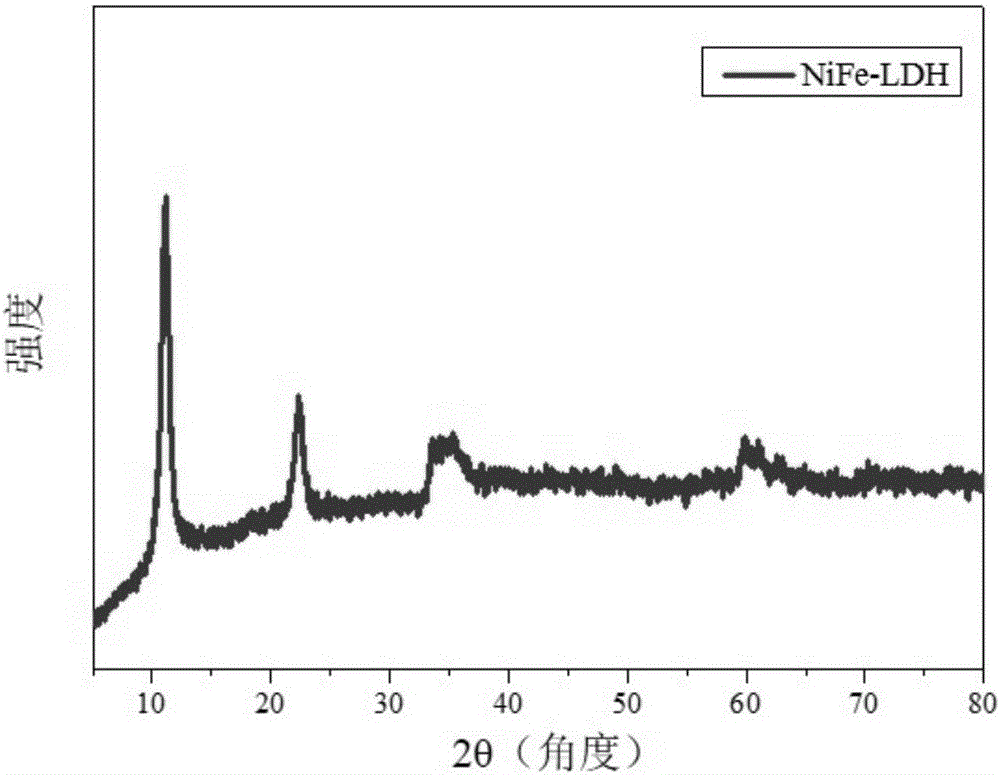
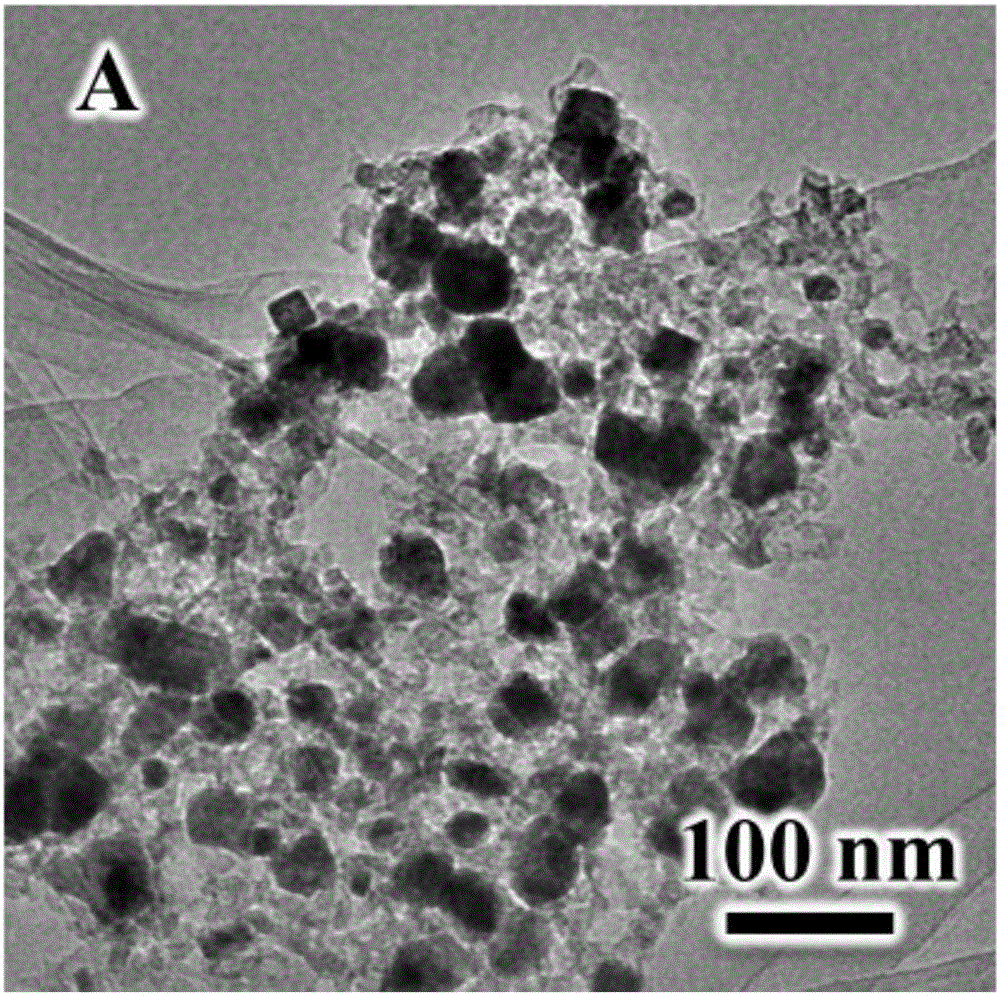
[0056] (1) Synthesis of NiFe-LDH precursor: 5 mL of n-butanol and 8 mL of oleylamine were placed in a reactor, stirred at high speed to obtain a microemulsion environment, and Ni 2+ , Fe 3+ Add it into the reactor at a certain molar ratio (1-3:1), mix it evenly, and then hydrothermally crystallize it in a highly closed system for 6-48 hours. The temperature of hydrothermal crystallization is 100-150°C. After the reaction, the product was centrifuged and washed with ethanol-water mixed solution, and dried to obtain NiFe-LDH. The chemical formula of the NiFe-LDH is [Ni 2+ 1-x Fe 3+ x (OH) 2 ] x+ ·(A n- ) x / n mH 2 O, 0.16≤x≤0.50, 0.5≤m≤9, its XRD is as follows figure 2 As shown, the NiFe-LDH has a size of 20-100 nm and a thickness of 3-10 nm.
[0057] (2) High-temperature nitriding: Put NiFe-LDH in an atmosphere tube electric furnace, program the temperature to ...
Embodiment 3
[0061] FeNi with ultrathin nanosheet structure 3 The preparation method of N electrocatalyst comprises:
[0062] (1) Synthesis of NiFe-LDH precursor: 5 mL of n-butanol and 8 mL of oleylamine were placed in a reactor, stirred at high speed to obtain a microemulsion environment, and Ni 2+ , Fe 3+ Add it into the reactor at a certain molar ratio (1-3:1), mix it evenly, and then hydrothermally crystallize it in a highly closed system for 6-48 hours. The temperature of hydrothermal crystallization is 100-150°C. After the reaction, the product was centrifuged and washed with ethanol-water mixed solution, and dried to obtain NiFe-LDH. The chemical formula of the NiFe-LDH is [Ni 2+ 1-x Fe 3+ x (OH) 2 ] x+ ·(A n- ) x / n mH 2 O, 0.16≤x≤0.50, 0.5≤m≤9, the size is 20-100nm, and the thickness is 3-10nm.
[0063] (2) High-temperature nitriding: place NiFe-LDH in an atmosphere tube electric furnace, program the temperature to 500°C in an ammonia atmosphere, and keep the high tempe...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com