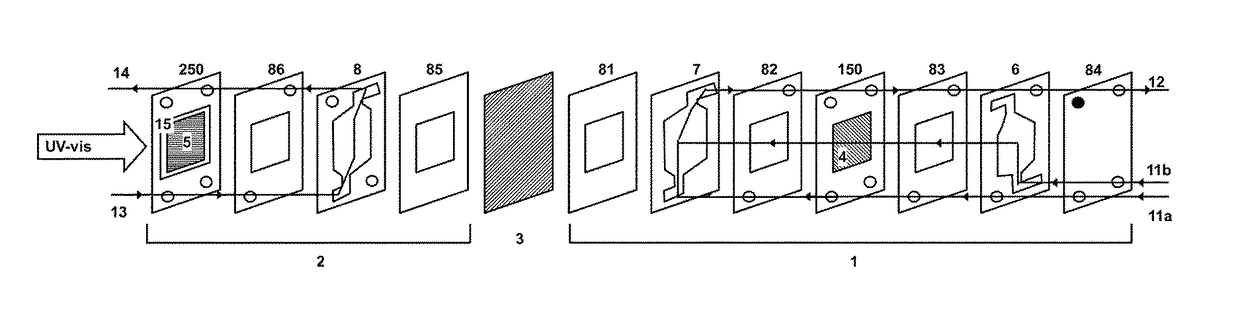
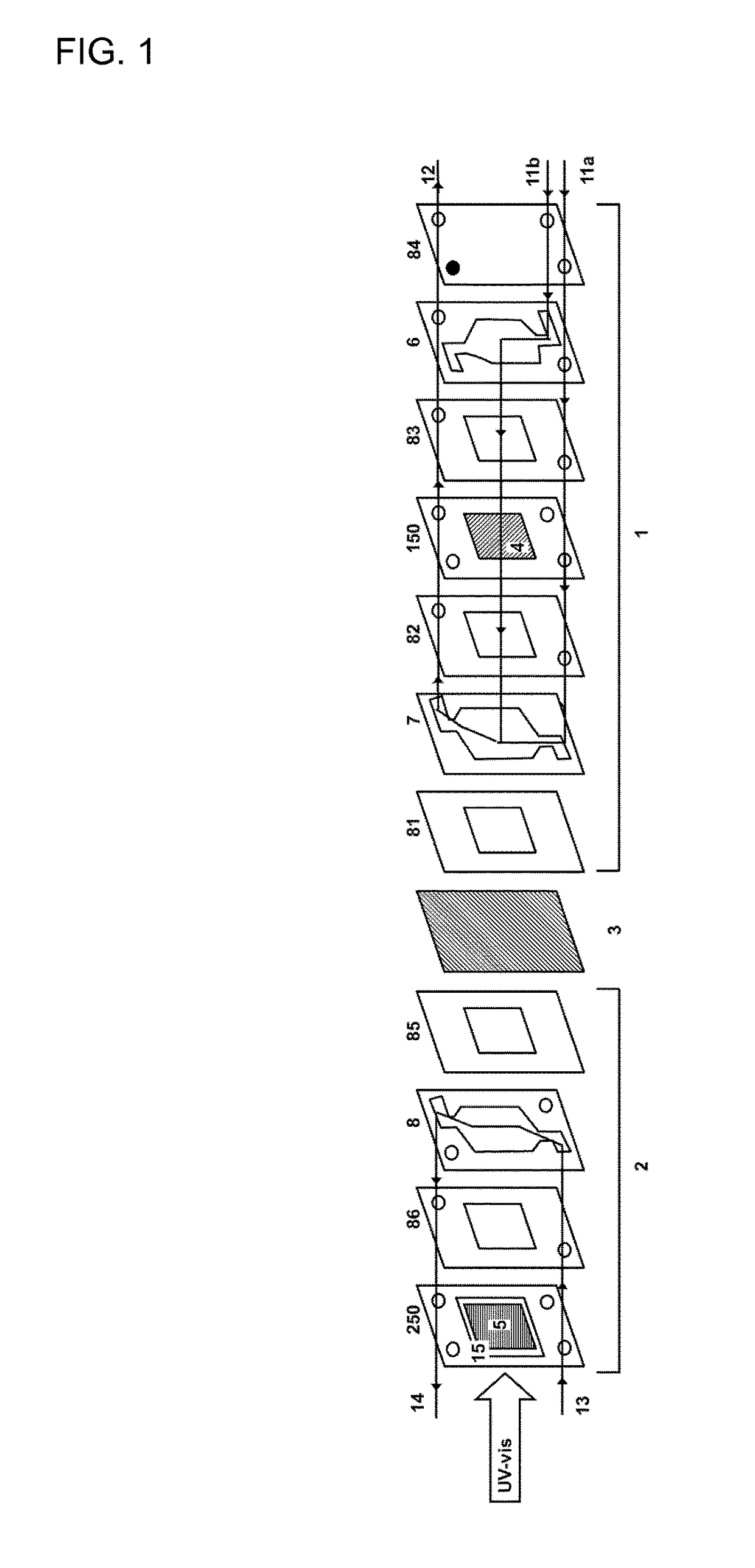
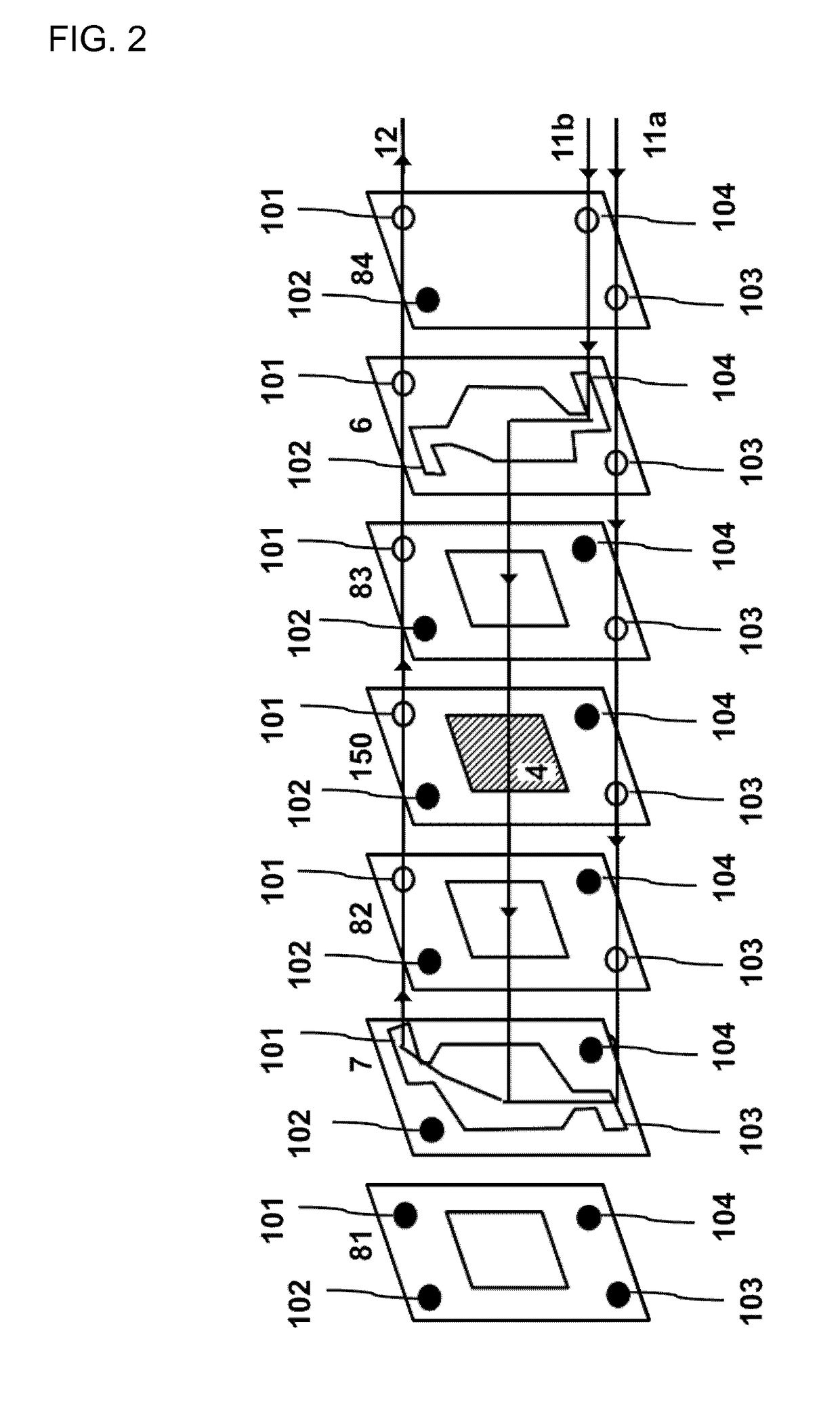
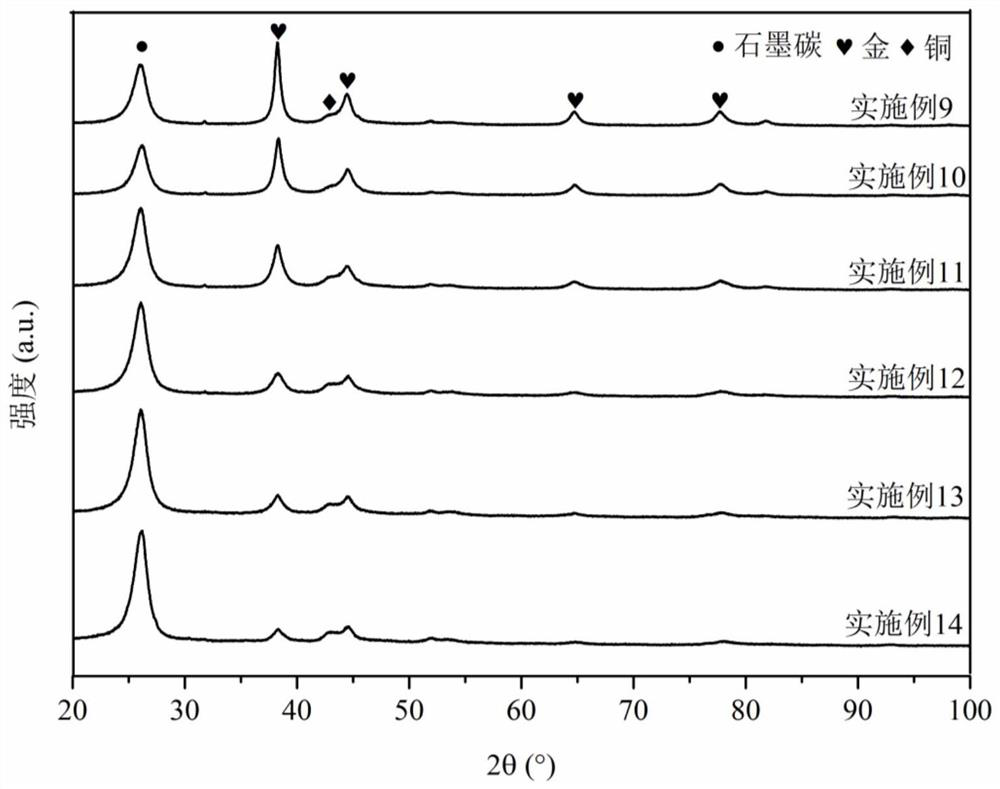
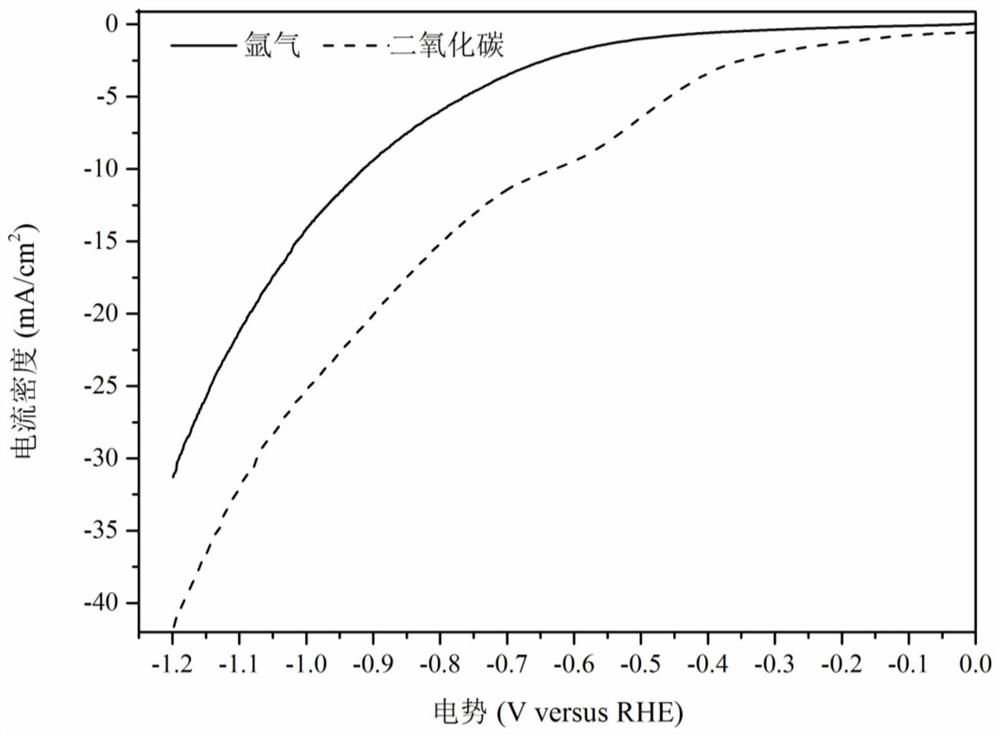
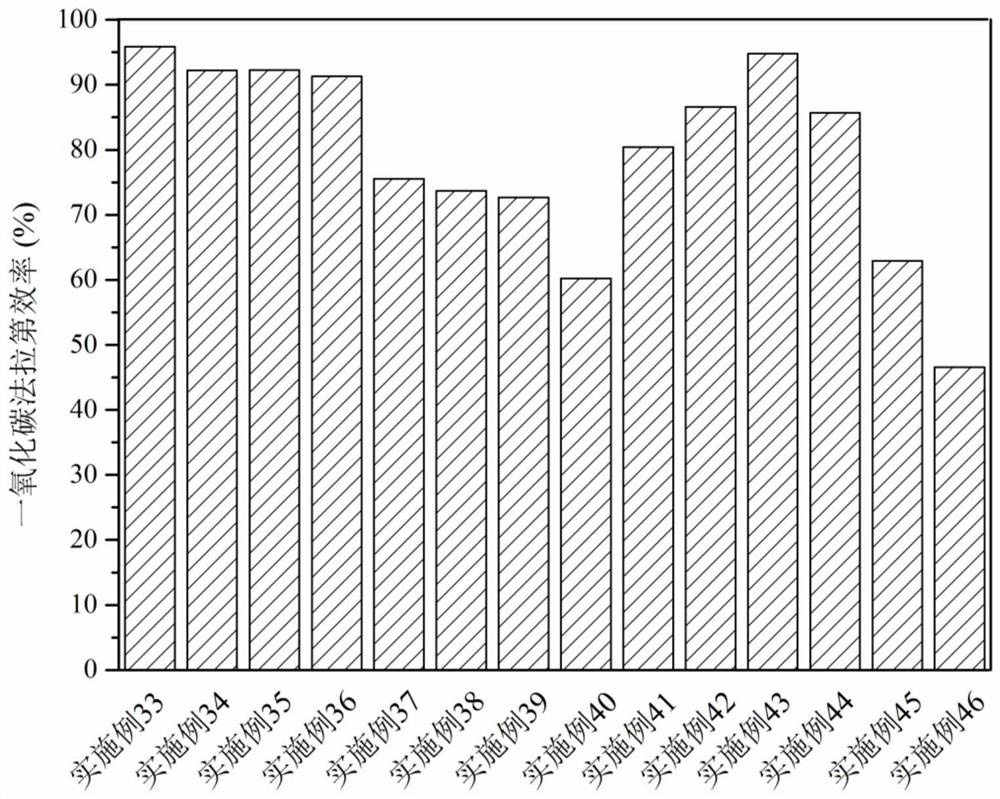
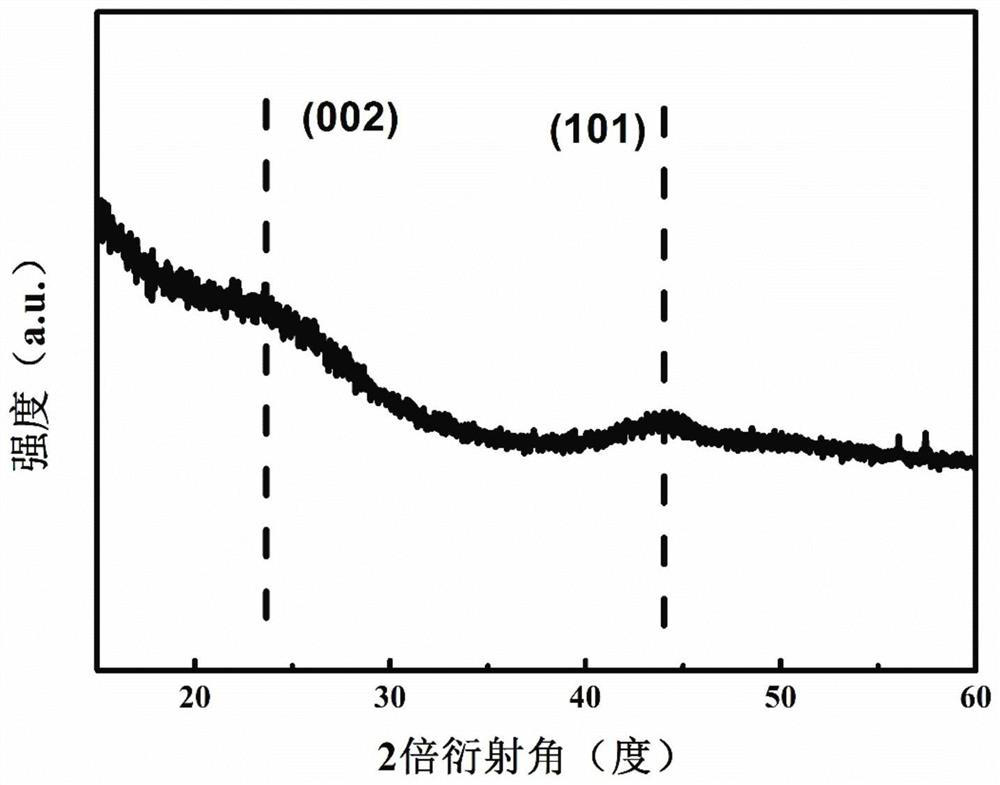
Patents
Literature
112results about How to "Improve Faraday efficiency" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Monatomic iron-nitrogen co-doped carbon electrocatalyst as well as preparation method and application thereof
InactiveCN111477889AHigh specific surface areaHigh selectivityCell electrodesBiochemical fuel cellsChemistryDoped carbon
The invention relates to the technical field of electrocatalysts. The invention discloses a monatomic iron-nitrogen co-doped carbon electrocatalyst as well as a preparation method and application thereof. Iron in the catalyst is wrapped in pores of a metal organic framework ZIF-8 containing a micropore and / or mesoporous structure in a monatomic iron-nitrogen coordination form, and the catalyst comprises micropores with pore diameters lower than 3nm, mesopores with pore diameters of 3-5nm and mesopores with pore diameters of 29-32nm. The preparation method of the vcomprises: (1) adding iron phthalocyanine into a 2-methylimidazole solution; (2) adding a zinc nitrate solution, and mixing to obtain a ZIF-8 packaged iron phthalocyanine composite material; and (3) calcining and pickling the composite material to obtain the electrocatalyst. The macroscopic morphology of the catalyst is a regular dodecahedron, the catalyst is regular and good in repeatability, and when the catalyst is applied,an electrolyte can achieve mass transfer through rich mesoporous structures in the catalyst, and high selectivity, high activity and low overpotential of CO preparation through electro-catalysis of CO<2> are achieved.
Owner:ZHEJIANG UNIV
Electrode for electrochemical reduction of CO2 and preparation of formic acid and preparation method and application thereof
ActiveCN104846397AIncrease current densityLow costElectrolytic organic productionElectrode shape/formsFaraday efficiencyReaction rate
The invention relates to an electrode for electrochemical reduction of CO2 and preparation of formic acid and a preparation method and an application thereof, and belongs to the field of a carbon dioxide resource technology. A traditional metal electrode has smooth surface and few catalytic active sites, which are not beneficial to rapid reaction. According to the invention, foamy copper with high specific surface is used as a substrate, and a layer of a low-melting-point metal catalyst is electroplated on the surface of the foamy copper so as to effectively raise reaction rate. The foamy copper can undergo surface electroplating in different formulations of electroplate liquids. The electroplated electrode still maintains a porous structure of foamy copper. The low-melting-point metal coating on the surface can efficiently electro-catalyze carbon dioxide to prepare formic acid. By the method, insufficiency of low surface catalytic active point of a traditional metal electrode can be improved. High Faradic efficiency of electrochemical reduction of CO2 and preparation of formic acid is guaranteed, and generation speed of formic acid also can be accelerated. The method is expected to be applied in the industrial process.
Owner:日照新睿招商发展有限公司
Method for preparing hydrocarbon through electrochemical reduction of carbon dioxide
InactiveCN105420751AImprove solubilityImprove Faraday efficiencyElectrolysis componentsElectrolytic organic productionElectrochemistryInorganic electrolyte
The invention relates to a method for preparing hydrocarbon through electrochemical reduction of carbon dioxide. An electrolytic tank is divided into a left cavity and a right cavity through a proton exchange membrane, the left cavity and the right cavity are not communicated with each other, and the two cavities serve as a cathode chamber and an anode chamber respectively; and the cathode chamber is filled with an electrolyte solution, the electrolyte solution is an inorganic electrolyte aqueous solution where the carbon dioxide is dissolved, and an organic solvent is further added into the electrolyte solution. The method of combining inorganic salts and the organic solvent is adopted, and metal Cu electrodes in different shapes and forms serve as cathodes, anodes are formed by Pt pieces or Pt wire electrodes and the like, and therefore the conversion rate of the carbon dioxide and the energy cycle utilization rate are greatly increased.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Method for electrocatalytic reduction of carbon dioxide using multi-metal oxygen cluster ionic liquid as electrocatalyst
ActiveCN104032324AEasy to synthesizeRaw materials are easy to getElectrolysis componentsOrganic-compounds/hydrides/coordination-complexes catalystsPhosphomolybdic acidResource utilization
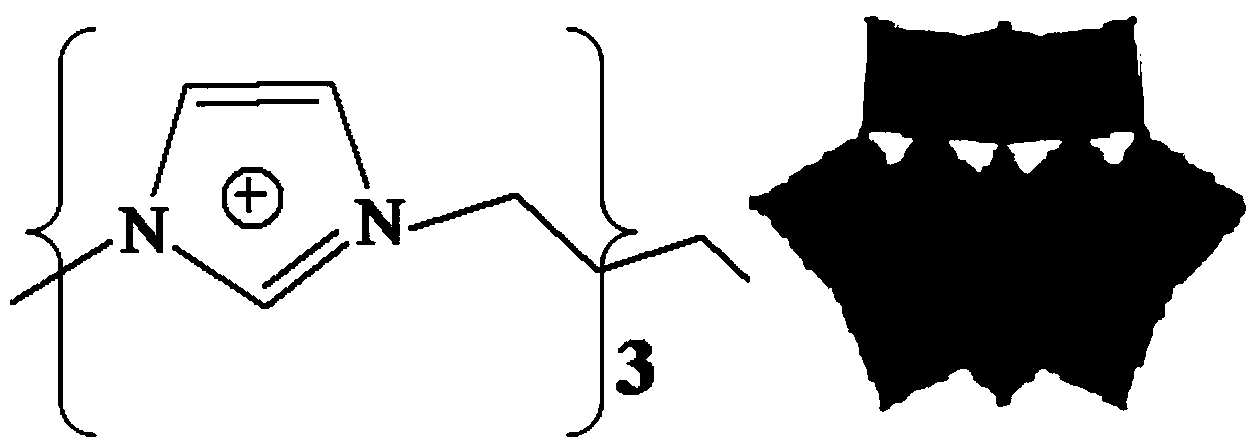
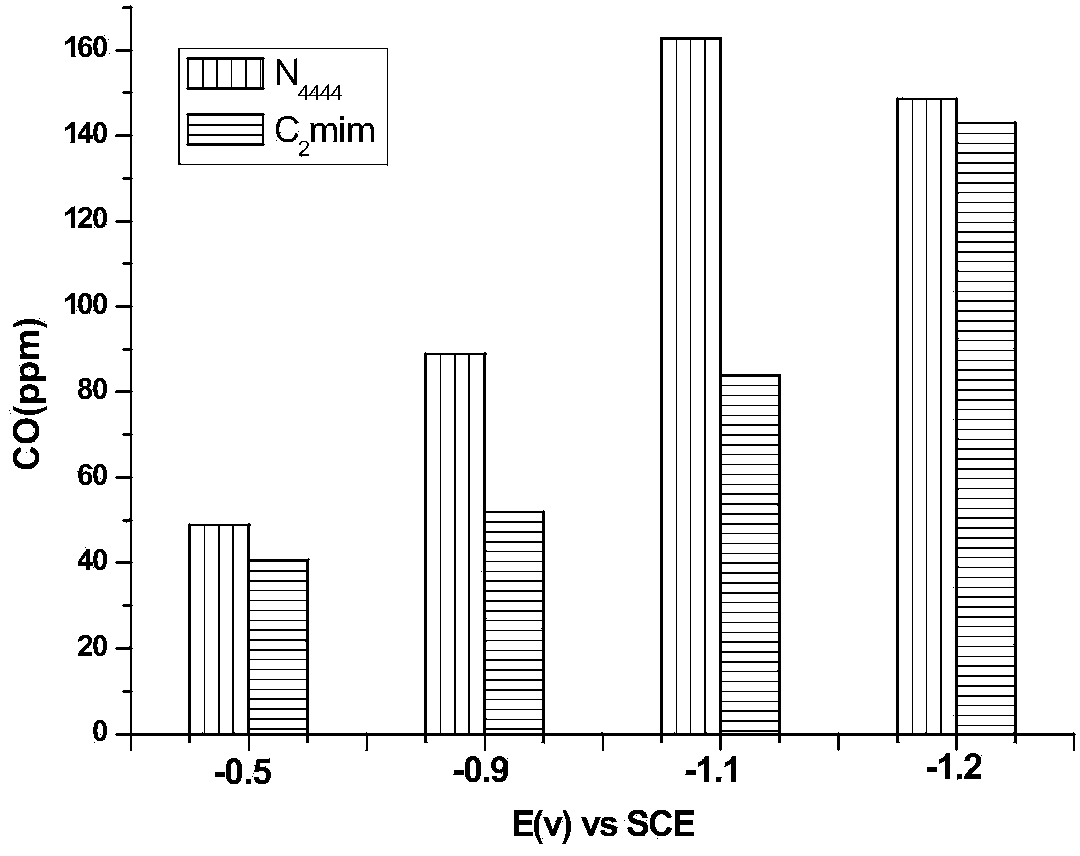
The invention relates to a method for electrocatalytic reduction of CO2 using multi-metal oxygen cluster ionic liquid as electrocatalyst. The method is characterized in that positive ions of the multi-metal oxygen cluster ionic liquid are imidazoles [Cnmim]+(n=2, 4, 6, 8), pyridines [Cnpyr]+(n=2, 4) and quaternary ammonium salts [N4444]+, and negative ions of the multi-metal oxygen cluster ionic liquid are phosphotungstic acid negative ions (PW12O403-) and phosphomolybdic acid negative ions (PMo12O403-); and the electrocatalytic reduction reaction is a three-electrode system, wherein a glassy carbon electrode is a working electrode, a platinum sheet electrode is a counter electrode, and a saturated calomel electrode is a reference electrode. The method is simple in process and convenient in operation, performs the electrocatalytic reduction for the CO2 to form such fuels as methane and carbon monoxide, realizes the resource utilization of the CO2, reduces the atmospheric pollution, and has important significance on the environmental protection.
Owner:INST OF PROCESS ENG CHINESE ACAD OF SCI
Preparation method of gas diffusion electrode for producing formic acid by electrochemical reduction of CO2
InactiveCN103741164AHigh mechanical strengthHigh electrical reduction efficiencyElectrolytic organic productionElectrode shape/formsWater bathsAcid washing
The invention relates to a preparation method of a gas diffusion electrode for producing formic acid by electrochemical reduction of CO2. The preparation method comprises the following steps: 1) adding anhydrous ethanol into powdery conductive carbon black till the powdery conductive carbon black is completely immersed, adding a polytetrafluoroethylene emulsion, performing reaction in a water bath, stirring till the formation of a lump, and then pressing by a roller to form a film, namely a diffusion layer film; 2) deoiling, acid-washing, tinning and drying a copper net to obtain a tinned copper net current collector; and 3) covering the diffusion layer film on the tinned copper net current collector, pressing by the roller, then cutting for formation, placing into a muffle furnace and calcining at the temperature of 3400 DEG C for 20-30min to prepare a target object. The preparation method provided by the invention has the advantages that according to the preparation method of the gas diffusion electrode, CO2 diffusion and mass transfer are strengthened by changing a CO2 transmission path and way of electrochemical reduction reaction of the CO2; and the preparation method has the advantages of simple process and low cost of raw materials, the prepared gas diffusion electrode has high mechanical strength, the electrochemical reduction efficiency of the CO2 is high, and the preparation method is suitable for engineering applications of electrochemical reduction of the CO2.
Owner:NANKAI UNIV
Method for preparing KA oil and derivatives of KA oil by electrocatalytic hydrogenation of lignin-based phenolic compounds
ActiveCN108660479AImprove conversion rateGood choiceElectrolytic organic productionChemical recyclingElectricityPlatinum
The invention relates to a method for preparing KA oil and derivatives by electrocatalytic hydrogenation of lignin-based phenolic compounds. According to the method, an H-shaped electrolytic cell is used as a container, in a negative pole chamber of the electrolytic cell, carbon fiber cloth is coated with a supported composite catalyst as a working electrode, and the lignin-based phenolic compounds are used as reaction substrates to be dissolved in an acidic solution as a negative pole solution; and a positive pole chamber uses a platinum sheet as a counter electrode and the acidic solution aspositive pole liquid, an electrocatalytic hydrogenation reaction is carried out at the temperature of 30-90 DEG C for 0.5-2 hours, and the KA oil and the derivatives of the KA oil are obtained afterpost-treatment. By the adoption of the method, by adopting the composite catalyst, the service life of the catalyst is greatly prolonged, and the conversion rate of the lignin-based phenolic compoundsreaches over 90-99%, the selectivity of the KA oil and the derivatives of the KA oil reaches over 90-95%, the Faraday efficiency can reach 80-90%, the cost is low, environmental protection is realized, the technological process is simple, the supported composite catalyst is recyclable, the production cost lowered, and the method has the high industrial value.
Owner:ZHEJIANG UNIV OF TECH
Method for preparing methane and ethylene through carbon dioxide electrochemical reduction
ActiveCN108588748AIncrease the catalytic activity of electrochemical reductionImprove Faraday efficiencyElectrolytic organic productionElectrodesFaraday efficiencyAuxiliary electrode
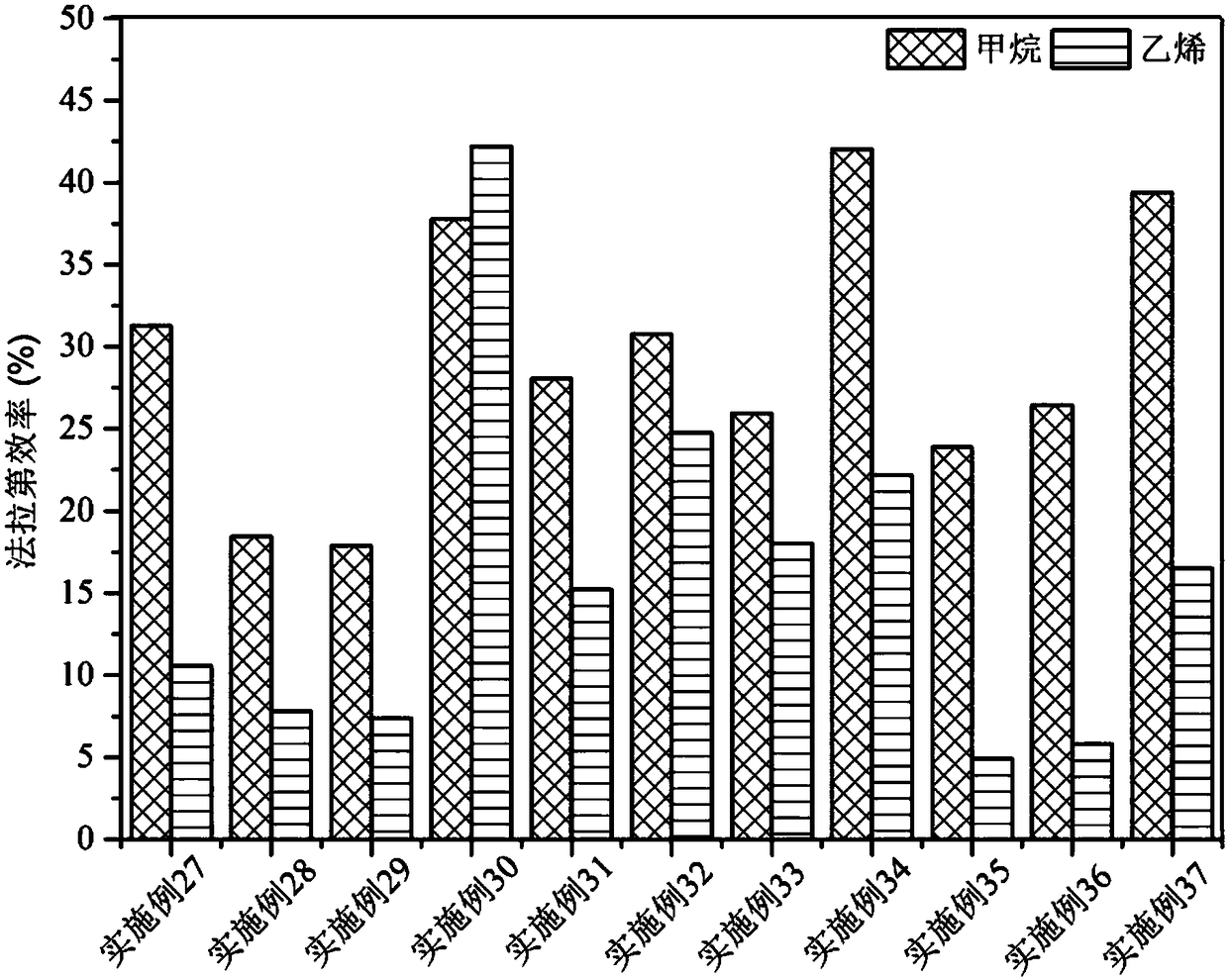
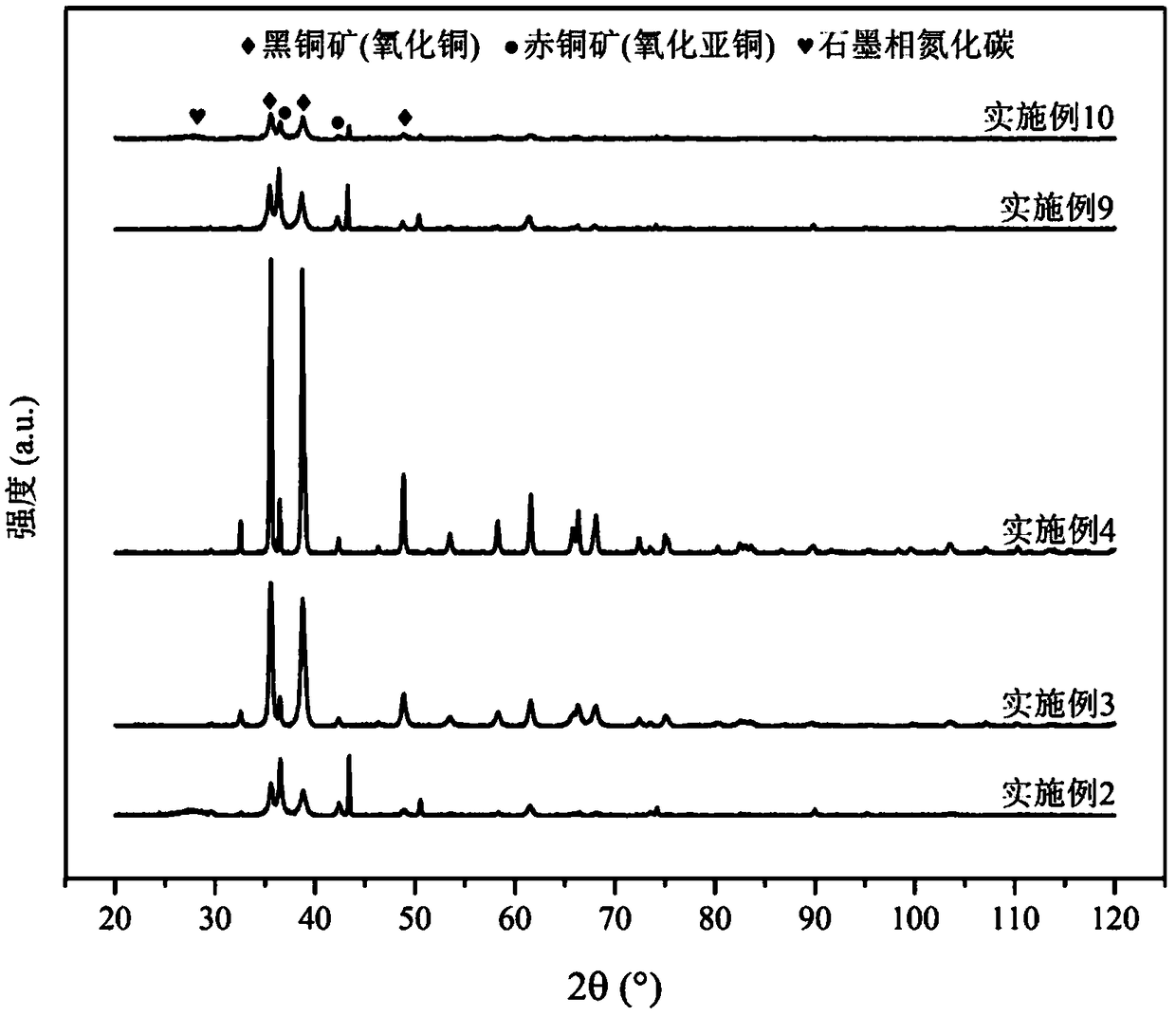
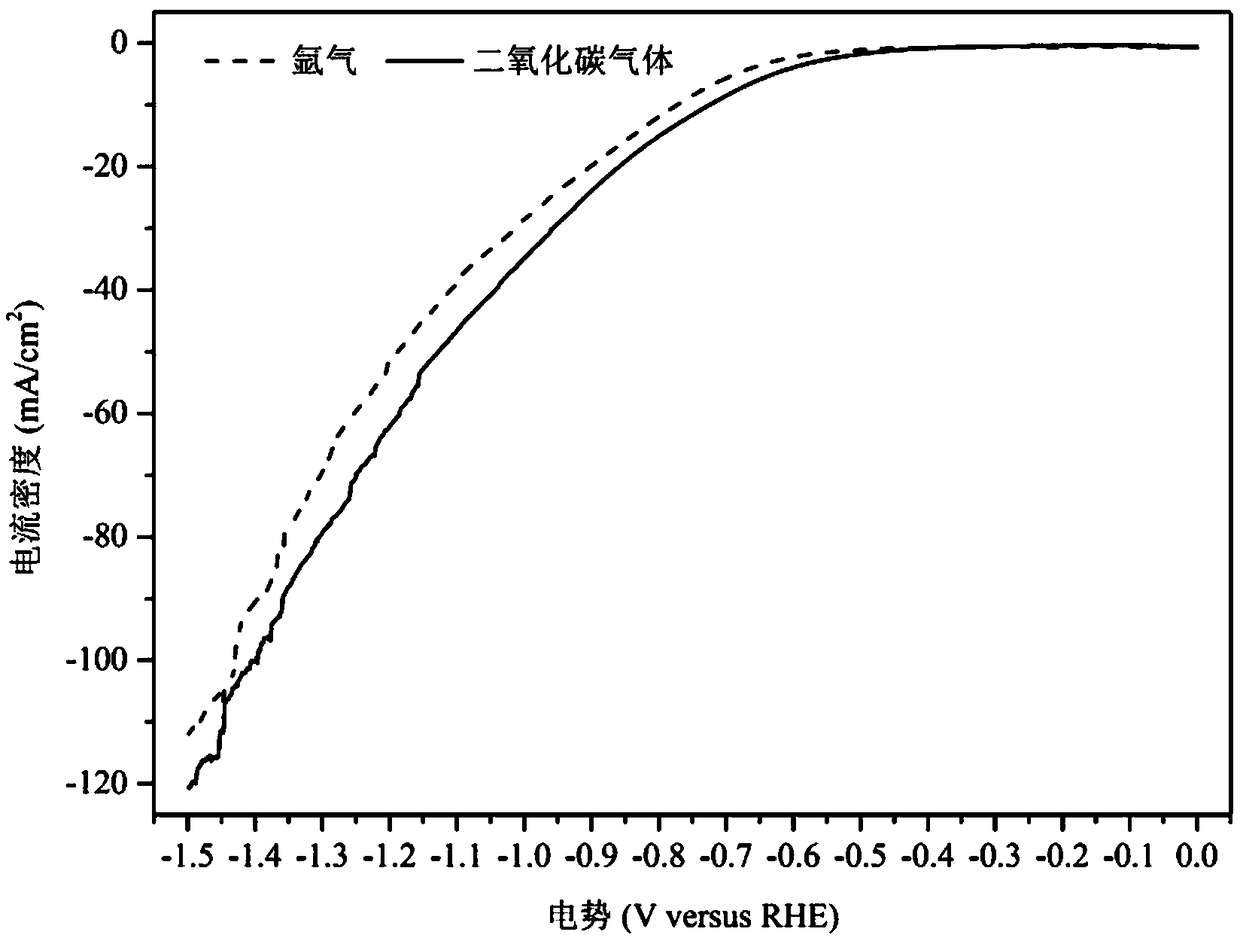
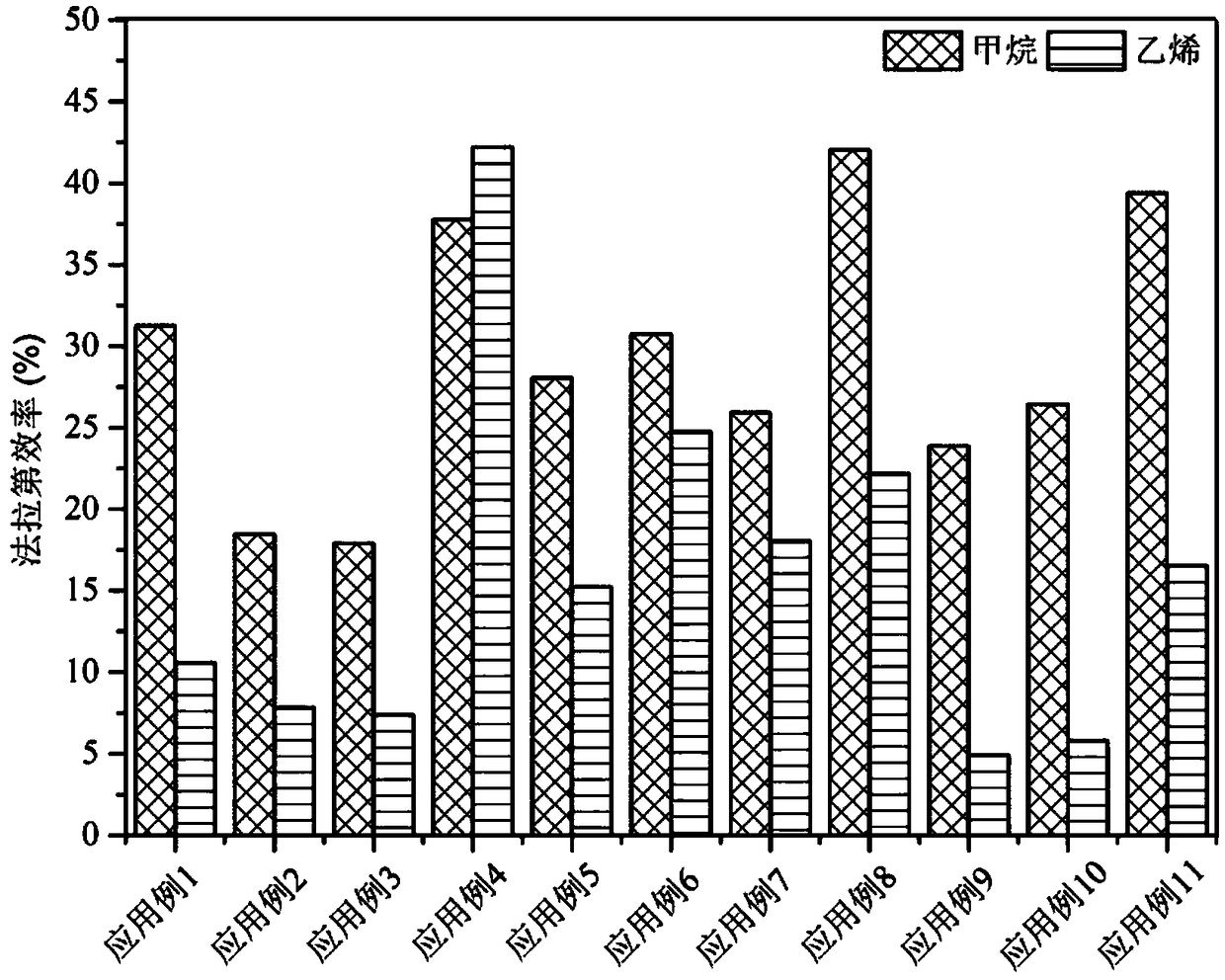
The invention relates to a method for preparing methane and ethylene through carbon dioxide electrochemical reduction. The method comprises the steps that an H-shaped dual-electrochemical-cell reactoris adopted, and is isolated into a cathode chamber and an anode chamber through a proton exchange membrane; before the reaction, carbon dioxide gas is led into the cathode chamber; a three-electrodesystem is adopted, a gas diffusion electrode serves as a work electrode, a platinum electrode serves as an auxiliary electrode, and a silver / silver chloride electrode serves as a reference electrode;the gas diffusion electrode comprises a gas diffusion electrode body and a carbon dioxide electrochemical reduction catalyst loaded gas diffusion electrode body; the carbon dioxide electrochemical reduction catalyst is graphite phase nitrogenated carbon supported nanometer copper oxide, and the nanometer copper oxide has tenorite and red copper ore. Through the method, the faradic efficiency of carbureted hydrogen of methane, ethylene and the like can be improved.
Owner:ZHEJIANG UNIV
Gas diffusion electrode, preparation method thereof and application thereof in electrochemical reduction of carbon dioxide
ActiveCN108823596AIncrease the catalytic activity of electrochemical reductionImprove Faraday efficiencyElectrolytic organic productionMetal/metal-oxides/metal-hydroxide catalystsFaraday efficiencyElectrochemical reduction of carbon dioxide
The invention relates to a gas diffusion electrode, a preparation method of the gas diffusion electrode, and the application of the gas diffusion electrode in electrochemical reduction of carbon dioxide. The gas diffusion electrode comprises a gas diffusion electrode body and carbon dioxide electrochemical reduction catalysts loaded on the gas diffusion electrode body. Nano copper oxide supportedon graphite phase carbon nitride serves as the carbon dioxide electrochemical reduction catalysts and has two crystal forms of tenorite and cuprit. The gas diffusion electrode can improve the Faradayefficiency of carbon dioxide electrochemical reduction products such as methane, ethylene and other hydrocarbons, and also can effectively inhibit hydrogen evolution reaction.
Owner:ZHEJIANG UNIV
Water-soluble fast reaction kinetics couple-based photoelectrochemical energy storage battery
ActiveCN106329033AEasy in situ transformationAdjustable capacityPhotoelectrochemical storage cellsElectrochemical responseExternal bias
The invention provides a water-soluble fast reaction kinetics couple-based photoelectrochemical energy storage battery. When the battery is charged, in-situ transformation of light energy into chemical energy is achieved by using photoelectrochemical reaction driven by self-bias of narrow band gap photoelectrodes and the chemical energy is stored into an active material of a battery electrolyte; and when the battery is discharged, electrochemical reaction is carried out, thereby achieving transformation of the chemical energy into electric energy. The photoelectrochemical energy storage battery integrates a photoelectrochemical battery and a flow battery; the disadvantage that a solar cell cannot achieve electric energy storage is overcome; meanwhile, a single charging mode of the energy storage battery is also expanded; and solar energy in-situ transformation, storage and controllable utilization without assistance of external bias are achieved. Water-soluble fast reaction kinetics redox couples are adopted as the active material; the utilization rate of photo-induced carriers on the surfaces of photoelectrodes is close to 100%; meanwhile, the discharge power density of the battery can reach 0.5W / cm<2>; large-scale amplification can be achieved; and the photoelectrochemical energy storage battery is suitable for different scales of solar energy-energy storage-power generation processes.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Catalyst for carbon dioxide reduction through electrocatalysis and preparation method thereof
InactiveCN110280283ALarge specific surface areaIncrease the specific surface area of contactPhysical/chemical process catalystsElectrolytic organic productionIndiumZinc sulfide
The invention belongs to the technical field of carbon dioxide reduction through electrocatalysis, and particularly relates to a catalyst for carbon dioxide reduction through electrocatalysis and a preparation method thereof. The catalyst is an indium zinc sulfide-MXene composite material. The catalyst has high electrocatalytic activity and selectivity on the carbon dioxide reduction, and significantly improves the energy efficiency for carbon dioxide utilization.
Owner:INT ACAD OF OPTOELECTRONICS AT ZHAOQING SOUTH CHINA NORMAL UNIV
Preparation method of electrode used for CO2 electrochemical reduction reaction
The invention relates to a preparation method of an electrode used for CO2 electrochemical reduction reaction. The electrode is prepared with foam copper, a copper wire mesh, a copper foil, a copper plate, a titanium wire mesh or a titanium plate as a substrate. The preparation method includes the steps of uniformly mixing a copper precursor solution being 0.01-2.0 M in concentrate and a template agent being 0.01-1.5 M in concentrate according to the molar ratio of 5:1-1:20 and magnetically stirring the solution for more than 30 min; moving the solution into a reaction kettle, immersing the substrate into the solution and performing a sealing reaction for 4-12 h; moving the substrate out from the reaction kettle, washing and drying the substrate, and performing thermal treatment to the substrate at 300-800 DEG C for 1-5 h under protection of an inert gas or an oxidizing atmosphere to obtain the substrate to which metal oxides are attached; and performing electrochemical reduction to the substrate to which metal oxides are attached in an acidic electrolyte to obtain the electrode. The preparation method is simple in preparation method and is suitable for large-scale production. The electrode is large in specific surface area and is high in CO2 oxygen reduction catalytic performance.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Method for synthesizing olefin by electro-catalyzing semi-hydrogenated gas-phase alkyne
PendingCN112301369AHigh selectivityGood choiceElectrolytic organic productionElectrodesChemical industryPtru catalyst
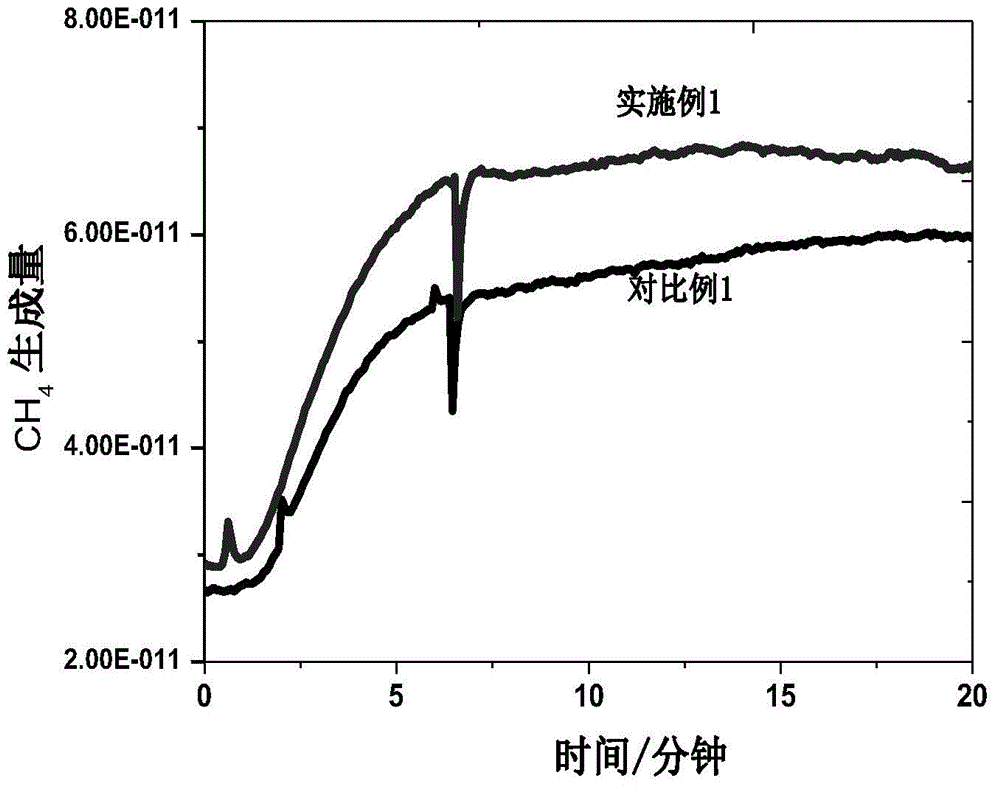
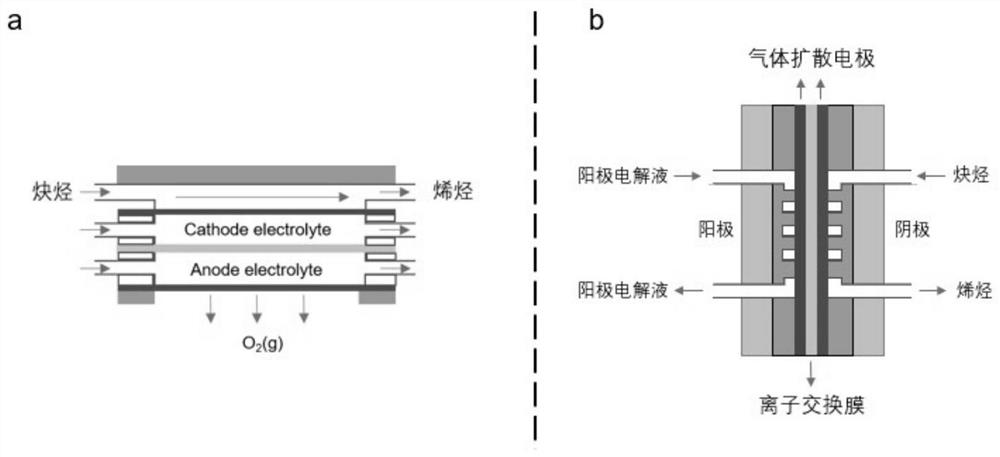
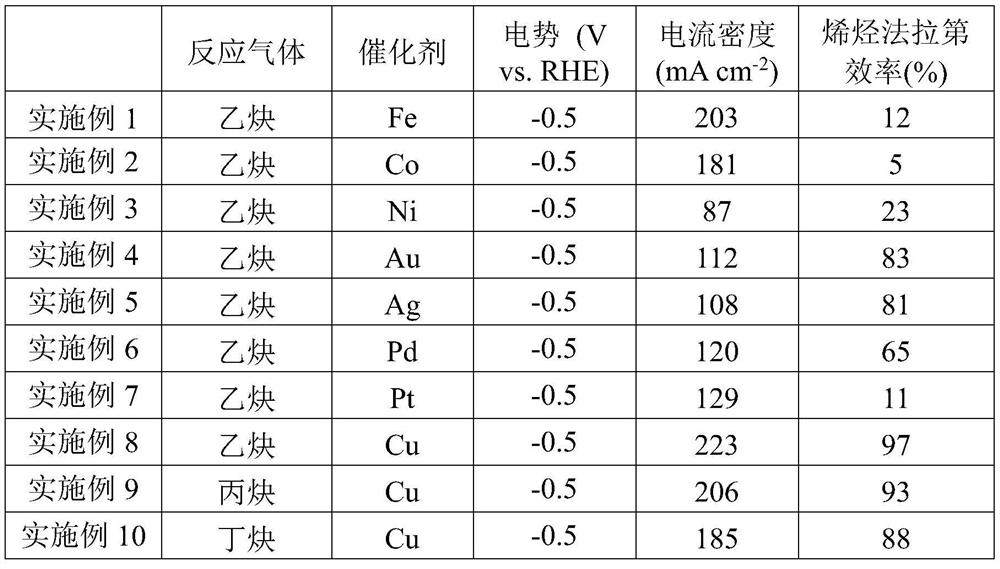
The invention relates to a method for synthesizing olefin by electro-catalyzing semi-hydrogenated gas-phase alkyne. The method comprises the following steps of: spraying a catalyst onto a gas diffusion layer substrate (comprising conductive carbon paper, metal and the like) by using a gas diffusion electrode, isolating a cathode from an anode by using an ion exchange membrane, and adopting a three-electrode or two-electrode system constant-voltage method to carry out electrochemical performance test, wherein the reaction gas is high-purity alkyne. According to the invention, the experimental results show that the method can be used for efficiently and selectively reducing the gas-phase alkyne into the corresponding olefin; compared with an H-type electrolytic tank, after the gas diffusionelectrode is used, the reaction current density is multiplied, the reaction voltage and the reaction current can reach 1 Acm<-2> or above by regulating and controlling the reaction voltage, and the Faraday efficiency of a target olefin product is remarkably improved and reaches 95% or above; and compared with the traditional thermal catalysis technology, the method can selectively reduce the gas-phase alkyne into olefin at normal temperature and normal pressure without hydrogen consumption, can greatly reduce the energy consumption in the process, better meets the requirements of green chemical industry, and has great strategic significance.
Owner:NORTHWESTERN POLYTECHNICAL UNIV
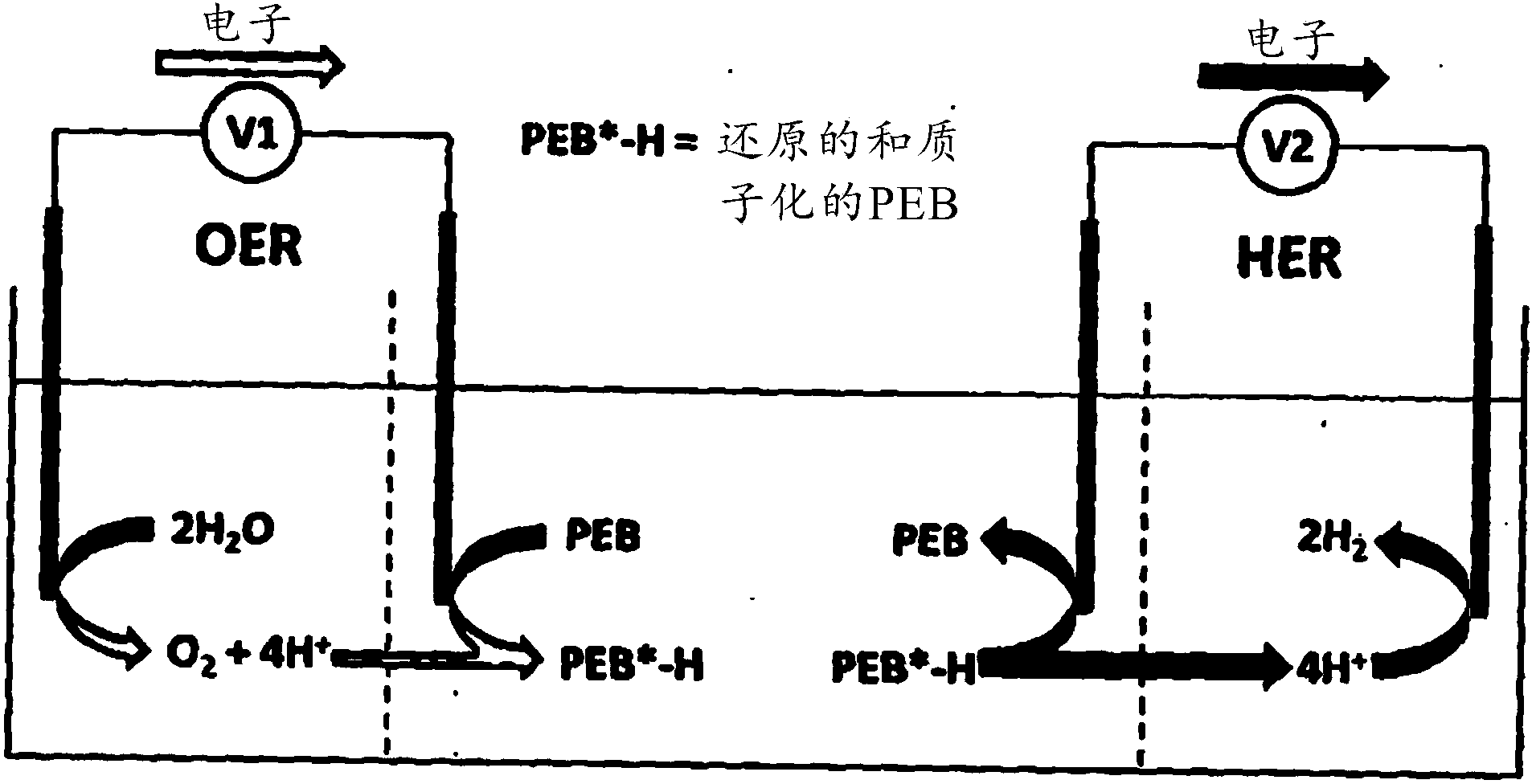
Apparatus and methods for electrochemical generation of oxygen and/or hydrogen
The invention provides methods for producing hydrogen and oxygen, comprising the steps of:(i) oxidising a mediator at a working electrode to yield an oxidised mediator, and reducing protons at a counter electrode to yield hydrogen; and (ii) reducing an oxidised mediator at a working electrode to yield a mediator, and oxidising water at a counter electrode to yield oxygen, wherein the oxygen generation step is performed non-simultaneously to the hydrogen generation step, and the oxidised mediator of step (i) is used as the oxidised mediator of step (ii), or the mediator of step (ii) is used as the mediator of step (i), and the mediator has a reversible redox wave lying between the onset of the oxygen evolution reaction (OER) and the hydrogen evolution reaction (HER).
Owner:格拉斯哥大学行政评议会
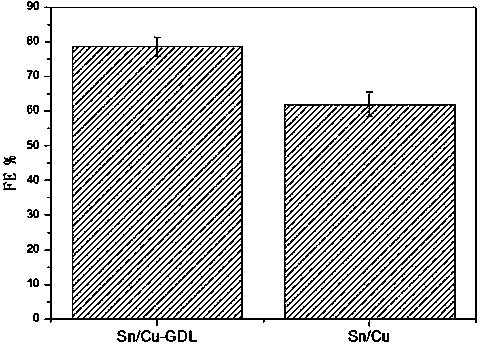
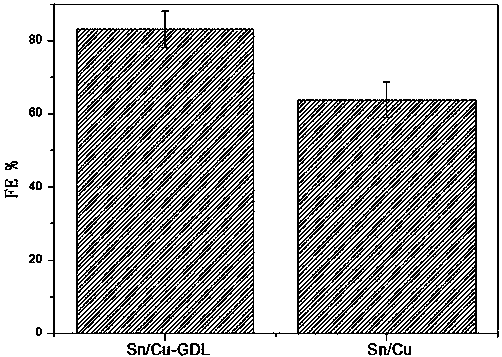
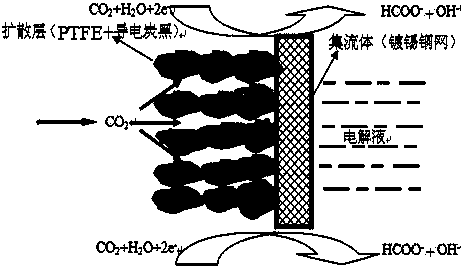
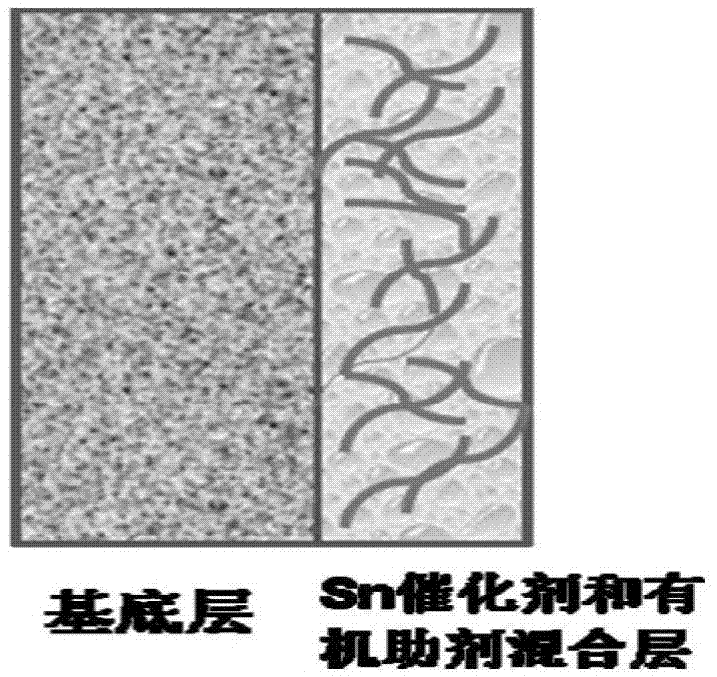
Gas diffusion electrode used for electrochemical reduction of carbon dioxide and preparation method and application of gas diffusion electrode
ActiveCN106876722AAvoid affecting performanceIncreased reaction current densityCell electrodesChemical reactionElectrochemical reduction of carbon dioxide
The invention relates to a gas diffusion electrode used for electrochemical reduction of carbon dioxide and a preparation method and an application of the gas diffusion electrode. The electrode comprises a base layer, and a Sn catalyst and an organic accessory ingredient mixing layer attached on the base layer; the molar ratio of the Sn catalyst to the organic accessory ingredient is 100:1 to 30:1; and the loading amount of the Sn catalyst in the electrode is 0.1-5mg / cm<-2>. In the electrochemical reduction of carbon dioxide, the base layer plays the effects of a supporting body, electric conduction, and forming liquid and gas transmission channels; the Sn catalyst catalyzes reduction of carbon dioxide while the organic accessory ingredient can stabilize an intermediate reactant CO<2>.-, so that the concentration of CO<2> is improved, overpotential in electrochemical reduction of carbon dioxide is lowered, and current density and efficiency are improved; and in addition, the accessory ingredient in the electrode also anchors the Sn catalyst on the surface of the carbon base, so that the electrode has quite high stability.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Filter-press photoelectrochemical water oxidation and co2 reduction cell
ActiveUS20180023203A1External energy supplyMaximizing CO selective conversionCellsCarbon compoundsPhotoelectrochemical cellGalvanic cell
The present disclosure relates to methods and devices for use in photoelectrochemical reduction of CO2. In particular, it is disclosed a filter-press photoelectrochemical cell for producing a reduction product from CO2 and a method for the photoelec-trochemical reduction of CO2.
Owner:SUNRGYZE SL
Graphene provided with polynitrogen coordination structure and preparation method and application thereof
ActiveCN110371957ALow costEfficient catalytic activityOrganic-compounds/hydrides/coordination-complexes catalystsGrapheneInfraredElectromagnetic shielding
The invention discloses graphene provided with a polynitrogen coordination structure and a preparation method and application thereof. The graphene contains 1-5 atomic layers, contains elements such as carbon, oxygen, nitrogen and hydrogen, and meanwhile contains at least one ofmetallic elements of iron, cobalt and nickel; the graphene has a catalytic active site of a polynitrogen coordination transition metal, and the coordination number of the nitrogen to metal is between 4 and 5, the graphene has efficient and stable monoatomic metal catalytic active site, and the graphene has high electrocatalytic performance for carbon dioxide, oxygen and formaldehyde. The graphene has the characteristics of easy dispersibility, easy processing and easy functioning, and functions of electric conduction, heat dissipation, infrared ray electric heating releasing and electromagnetic shielding are easily achieved.
Owner:ANHUI UNIVERSITY
Iron-based non-noble metal catalyst for electrocatalytic synthetic ammonia and preparation method thereof
The invention discloses an iron-based non-noble metal catalyst for electrocatalytic synthetic ammonia and a preparation method thereof. The preparation method is characterized in that bivalent iron ions in the bivalent iron salt solution are reduced and deposited on the surface of a conductive carrier by adopting an electrochemical deposition method, and the bivalent iron salt solution contains bivalent iron salt, a reducing agent, o-sulfonyl imide and sodium dodecyl sulfate. According to the preparation method, the catalyst is carried out electro-catalysis to synthesize ammonia, so that the faraday efficiency of the electrocatalysis synthetic ammonia process can be obviously improved.
Owner:SHANDONG NORMAL UNIV
Nanometer tin based catalyst for electrochemical reduction carbon dioxide reaction and preparation method and application thereof
InactiveCN108441878ALarge specific surface areaRich grain boundary structureMaterial nanotechnologyElectrolytic organic productionTin dioxideNanowire
The invention discloses a nanometer tin based catalyst for an electrochemical reduction carbon dioxide reaction and a preparation method and application thereof. The nanometer tin based catalyst is ofa one-dimensional structure composed of a stannic oxide nanotube and a stannic oxide nanowire. The stannic oxide nanotube is arranged on the outer portion of the stannic oxide nanowire in a sleevingmanner. The nanometer tin based catalyst is prepared and obtained through combination of the one-step electrostatic spinning process and calcining treatment. The nanometer tin based catalyst in the special shape can be used for catalyzing the electrochemical reduction carbon dioxide reaction, and the Faradic efficiency and the current density of the reaction can be obviously improved.
Owner:ZHEJIANG UNIV
Method for preparing hydrogen sulfide through electrochemical reduction of sulfur dioxide
ActiveCN113122864AEasy to separateImprove Faraday efficiencyCellsGas treatmentColloidElectrochemistry
The invention discloses a method for preparing hydrogen sulfide through electrochemical reduction of sulfur dioxide. According to the method, sulfur dioxide absorption liquid is subjected to electrochemical reduction through a membrane electrode to generate hydrogen sulfide gas. The method can realize high-efficiency and high-selectivity conversion of the sulfur dioxide absorption liquid into hydrogen sulfide, so that colloidal sulfur which is generated on the cathode and adhered to the surface of the cathode can be prevented from poisoning the cathode, and the method does not need to additionally add a reducing agent, does not generate waste salt, can be carried out under normal-temperature and normal-pressure conditions, and is simple to operate and convenient for large-scale application.
Owner:CENT SOUTH UNIV
Catalyst for electrocatalytic carbon dioxide reduction reaction as well as preparation method and application of catalyst
ActiveCN113403644AImprove Faraday efficiencyElectrolytic organic productionElectrodesPtru catalystFaraday efficiency
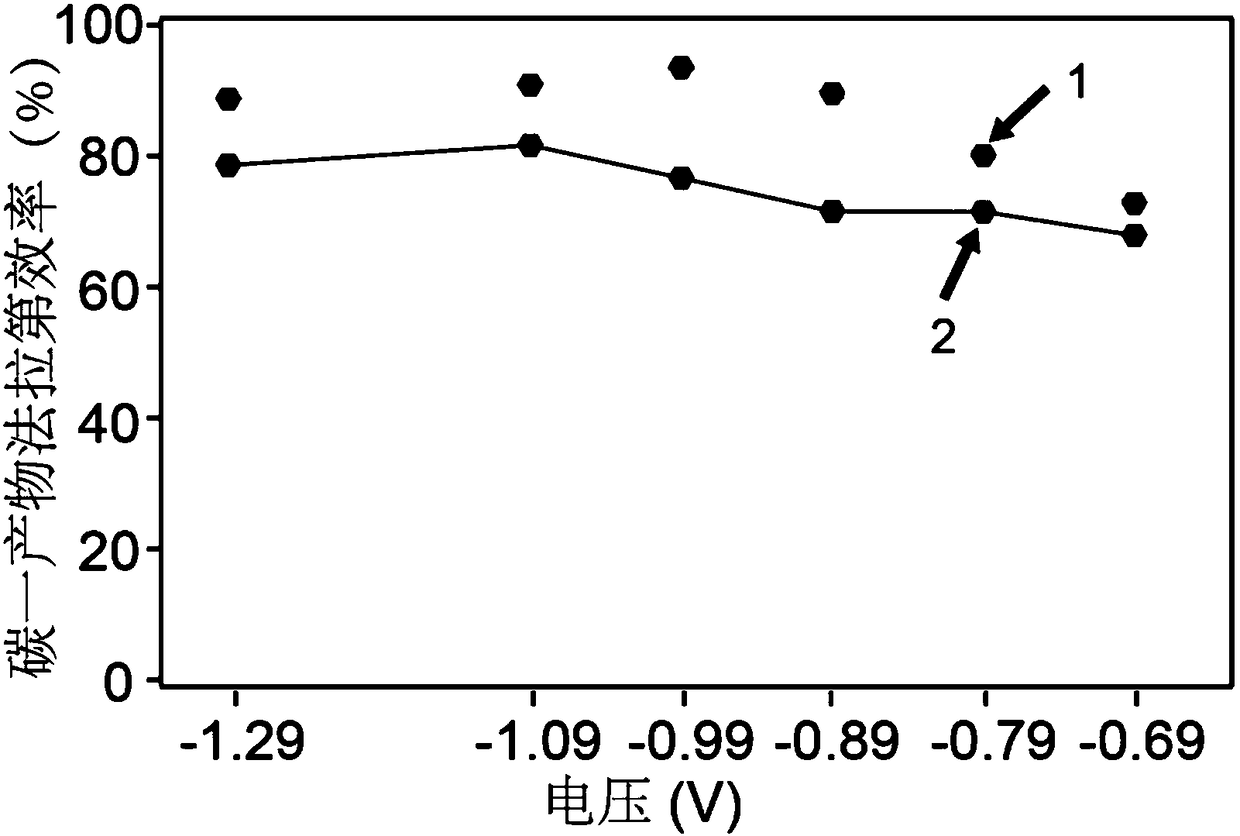
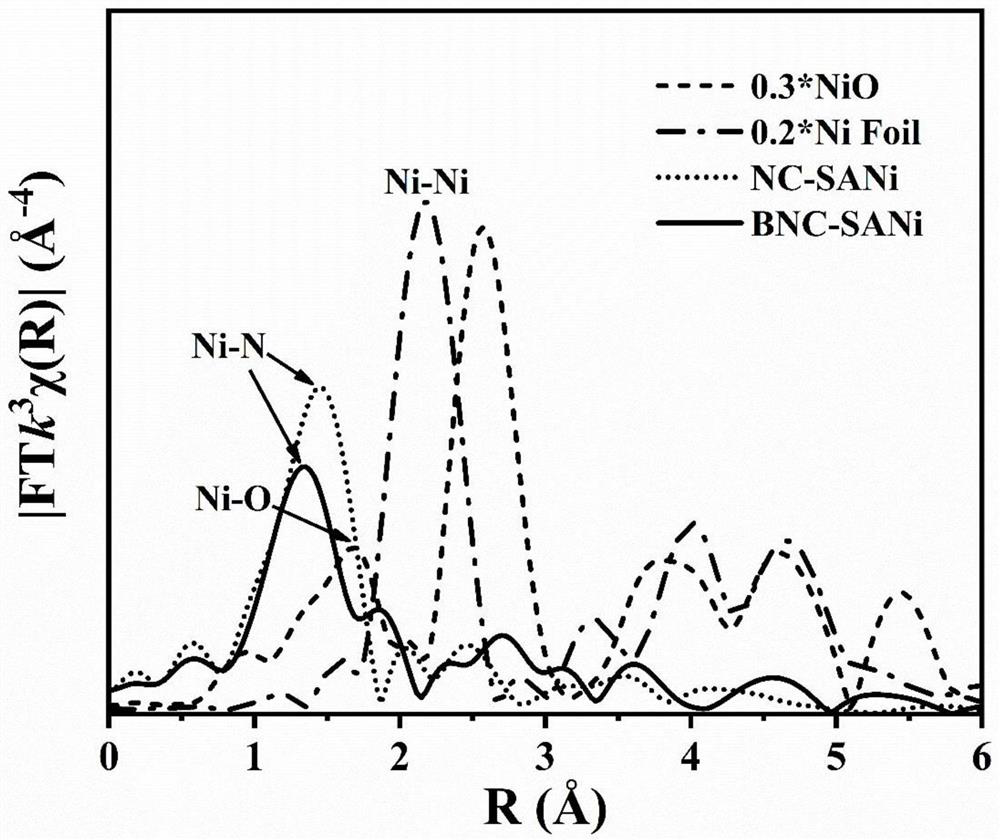
The invention belongs to the technical field of catalyst preparation, and provides a catalyst for electrocatalytic carbon dioxide reduction reaction as well as a preparation method and application thereof. According to the catalyst disclosed by the invention, a boron element is doped on a three-dimensional porous carbon skeleton, and the boron element, a nitrogen atom and a nickel metal single atom form a Ni-N4B2 active site, that is, the doping of the boron element changes a coordination environment of the center of the active site; therefore, the catalyst improves the Faraday efficiency of the product of the Ni-N4 site under high current density in the electrocatalysis carbon dioxide reduction process. Data of the embodiment show that when the catalyst obtained in the embodiment 1 is loaded on a gas diffusion electrode, the current density of the catalyst reaches 213 + / -30mA. Cm <-2 > under the potential of-1.2 V, and the Faraday efficiency of the product carbon monoxide under the potential reaches up to 97.37%.
Owner:BEIHANG UNIV
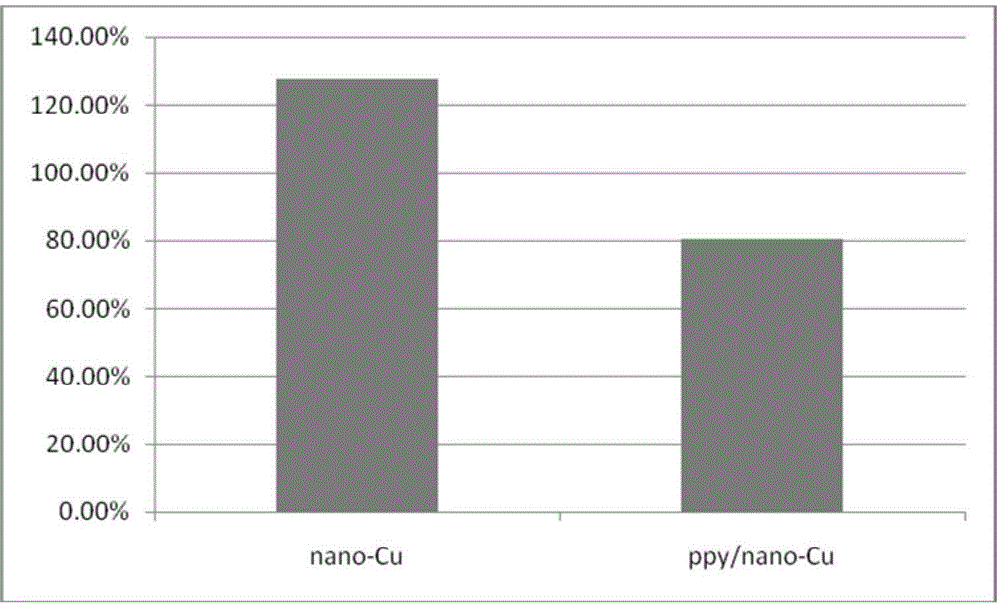
Polypyrrole/nano-copper composite gas diffusion electrode and preparation and application thereof
InactiveCN105316702AImprove Faraday efficiencyImprove conversion rateElectrolytic organic productionElectrodesPolypyrroleFaraday efficiency
The invention relates to a polypyrrole / nano-copper composite gas diffusion electrode and preparation and an application thereof. Carbon paper or carbon cloth serves as a substrate, a polypyrrole layer with the thickness being 0.05-1.0 [mu]m is attached to the surface of the substrate, and a nano-copper material layer with the thickness being 0.1-1.0 [mu]m is deposited on the polypyrrole layer. Polypyrrole is aggregated and nano-copper is deposited on the carbon-based electrode, the hydrogen evolution side reaction is reduced, and the Faradic efficiency of products is improved and the conversion rate of carbon dioxide is increased.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Preparation method and application of rare earth metal and transition metal co-doped carbon-based material
The invention discloses a preparation method and application of a rare earth metal and transition metal co-doped carbon-based material; the method includes: weighing a proper amount of porous carbon, a transition metal precursor and a rare earth metal precursor, adding deionized water and ethanol, and after ultrasonic treatment is performed for 30 min, preparing a mixed solution A; then adding the mixed solution A into the organic ligand solution, and stirring at room temperature for 3-5 hours; centrifuging, washing and drying the mixture; and putting the obtained powder into a tubular furnace for heat treatment, carrying out acid pickling treatment on the obtained black powder, then carrying out suction filtration and water washing until the filtrate is neutral, and drying the powder to obtain the transition metal and rare earth metal co-doped carbon-based material. The prepared carbon-based material has rich pore structures and excellent carbon monoxide selectivity, the Faraday efficiency can reach 99% at most, the current density can reach 97 mA / cm2 at most, and the electrochemical performance is excellent.
Owner:CHINA UNIV OF MINING & TECH
Catalyst for carbon dioxide reduction through electrocatalysis and preparation method thereof
InactiveCN110280294AIncrease the specific surface area of contactImprove Faraday efficiencyPhysical/chemical process catalystsChemical industryNanofiberIron sulfide
The invention belongs to the technical field of carbon dioxide reduction through electrocatalysis, and particularly relates to a catalyst for carbon dioxide reduction through electrocatalysis and a preparation method thereof. The catalyst is nitrogen-doped carbon nanofiber composite iron sulfide particles. The catalyst has high electrocatalytic activity and selectivity on the carbon dioxide reduction, in particular, can significantly improve the energy efficiency for carbon dioxide utilization; in addition, the preparation method of the catalyst has the advantages of simple operation, environmental friendliness, high yield, and broad application prospects.
Owner:INT ACAD OF OPTOELECTRONICS AT ZHAOQING SOUTH CHINA NORMAL UNIV
Preparation of nano-flaky iron-doped nickel phosphide and nitrogen reduction reaction (NRR) application
InactiveCN109876835AImprove efficiencyGood effectMaterial nanotechnologyCatalyst activation/preparationElectrolysisFaraday efficiency
In view of the heavy demand for ammonia and the harsh reaction condition and low conversion rate of a Haber-Bosch process, simple preparation of the ammonia becomes a major problem of development of the present world, and therefore, the study of achieving nitrogen reduction ammonia through electrolysis of an electrolyte with saturated nitrogen at the normal temperature and normal pressure is paidmuch attention. The invention provides a preparation method of nano-flaky iron-doped nickel phosphide nano-powder and application of the nano-flaky iron-doped nickel phosphide nano-powder to a nitrogen reduction reaction (NRR). The preparation method comprises the steps: firstly, a specific proportion of iron source reagent and nickel source reagent are added into a reaction solution, a heating reaction is conducted, and iron-nickel precursor nano-powder is obtained; and then the iron-nickel precursor nano-powder is placed into a tube furnace at the specific nitrogen flow rate to be subjectedto a phosphorization reaction, and finally the nano-flaky iron-doped nickel phosphide nano-powder is obtained. The nano-flaky iron-doped nickel phosphide nano-powder shows excellent catalytic activityin the field of the NRR, the ammonia yield under minus 0.3 V (relative to a standard hydrogen electrode) reaches up to 70.6 [mu]g h<-1> mg<cat.><-1>, and the Faradic efficiency reaches 6.5%.
Owner:UNIV OF JINAN
Method for preparing carbon monoxide through carbon dioxide electrochemical reduction
ActiveCN112251766AIncrease the catalytic activity of electrochemical reductionImprove Faraday efficiencyCellsElectrodesPtru catalystFaraday efficiency
The invention relates to a method for preparing carbon monoxide by electrochemical reduction of carbon dioxide. The method comprises the following steps of: separating a cathode chamber and an anode chamber by a proton exchange membrane by adopting an H-shaped double-electrochemical-cell reactor; introducing carbon dioxide gas into the cathode chamber before reaction; and using a three-electrode system, wherein a gas diffusion electrode is used as a working electrode, a platinum electrode is used as an auxiliary electrode, a silver / silver chloride electrode is used as a reference electrode, the gas diffusion electrode comprises a gas diffusion electrode body and a carbon dioxide electrochemical reduction catalyst loaded on the gas diffusion electrode body, and the carbon dioxide electrochemical reduction catalyst is gold-based bimetal supported by multi-walled carbon nanotubes. The method can improve the Faraday efficiency of the product carbon monoxide.
Owner:SINOPEC NANJING RES INST OF CHEM IND CO LTD +2
Preparation method and application of carbon material rich in topological defects obtained through high-temperature ammonia treatment
ActiveCN112830468AHow many topological defectsSimple manufacturing methodPhysical/chemical process catalystsDispersed particle separationPtru catalystPhysical chemistry
The invention discloses a preparation method for obtaining a carbon material rich in topological defects through high-temperature ammonia treatment. According to the method, a nitrogen-doped carbon material is used as a precursor, heat treatment is carried out in an ammonia atmosphere, and the carbon material rich in carbon topological defects is obtained, has efficient carbon dioxide electro-reduction catalytic activity, has high selectivity on CO and has high stability, so that the carbon material can be used as a carbon dioxide electrochemical reduction catalyst.
Owner:NINGBO INST OF MATERIALS TECH & ENG CHINESE ACADEMY OF SCI +1
Boron-doped diamond supported metal single atom and preparation method and application thereof
ActiveCN110560034AImprove Faraday efficiencyImprove atom utilization efficiencyCatalyst activation/preparationMetal/metal-oxides/metal-hydroxide catalystsMetallurgyElectrochemical window
The invention provides a boron-doped diamond supported metal single atom. The boron-doped diamond supported metal single atom comprises a substrate, a boron-doped diamond thin film arranged on one ortwo sides of the substrate, and a catalyst uniformly supported on the surface of the boron-doped diamond thin film, wherein the catalyst comprises a metal single atom. A prepared electrode of the boron-doped diamond supported metal single atom has a wide electrochemical window, high stability, and high catalytic activity and product selectivity, so that selectivity and efficiency of a catalytic process are greatly improved.
Owner:SHENZHEN INST OF ADVANCED TECH
Preparation method of defective graphene anchored double transition metal monatomic synthesis ammonia catalyst
ActiveCN113235123AIncreased single-atom loading rateHigh catalyst activityElectrodesDefective graphenePolystyrene microsphere
The invention discloses a preparation method of a defective graphene anchored double transition metal monatomic synthesis ammonia catalyst, and the preparation method comprises the following steps: synthesizing polystyrene microspheres, centrifugally washing, and drying in vacuum; stirring and adsorbing the polystyrene microspheres and a transition metal salt solution; stirring the polystyrene microspheres adsorbing the transition metal salt with a defective graphene oxide aqueous solution to form a composite structure taking the polystyrene microspheres as a core and the defective graphene oxide as a shell, performing centrifuging, and performing drying in vacuum; dispersing the sample in an aqueous solution, adding a small amount of another transition metal salt solution, carrying out ultrasonic dispersion, performing freeze-drying, and carrying out high-temperature annealing to obtain a final product. According to the invention, polystyrene microspheres are utilized to adsorb a first transition metal salt, so the first transition metal salt is distributed in an inner shell layer of defective graphene oxide; a second transition metal salt is loaded by utilizing a defective graphene oxide shell layer, and the polystyrene microspheres are removed by utilizing high-temperature annealing to form a structure that the defective graphene hollow inner shell layer and the defective graphene hollow shell layer are respectively loaded with two double-transition metal monatomic structures.
Owner:CHINA THREE GORGES UNIV
Carbon quantum dot catalyst applied to preparation of methane by electroreduction of carbon dioxide and preparation method of carbon quantum dot catalyst
PendingCN113604833AGood choiceImprove Faraday efficiencyElectrolytic organic productionElectrodesCarbon quantum dotsMethane
The invention discloses a carbon quantum dot catalyst applied to preparation of methane by electroreduction of carbon dioxide and a preparation method of the carbon quantum dot catalyst, and belongs to the technical field of carbon quantum dot catalysts for preparation of methane by electroreduction of carbon dioxide. A uric acid compound is selected as a precursor and an alkaline solution, and carbon quantum dots are obtained through a top-down molecular fusion method. The synthesized carbon quantum dots have efficient selectivity and high Faraday efficiency for methane preparation. The used raw materials are low in price and easy to obtain, the preparation process is simple, the cost is low, carbon dioxide can be efficiently catalyzed and electrochemically reduced into methane, resource utilization of carbon dioxide can be effectively achieved, the carbon cycle industry chain is perfected, and good application prospects are achieved.
Owner:SHANGHAI UNIV
Application of BN to electrochemical ammonia production
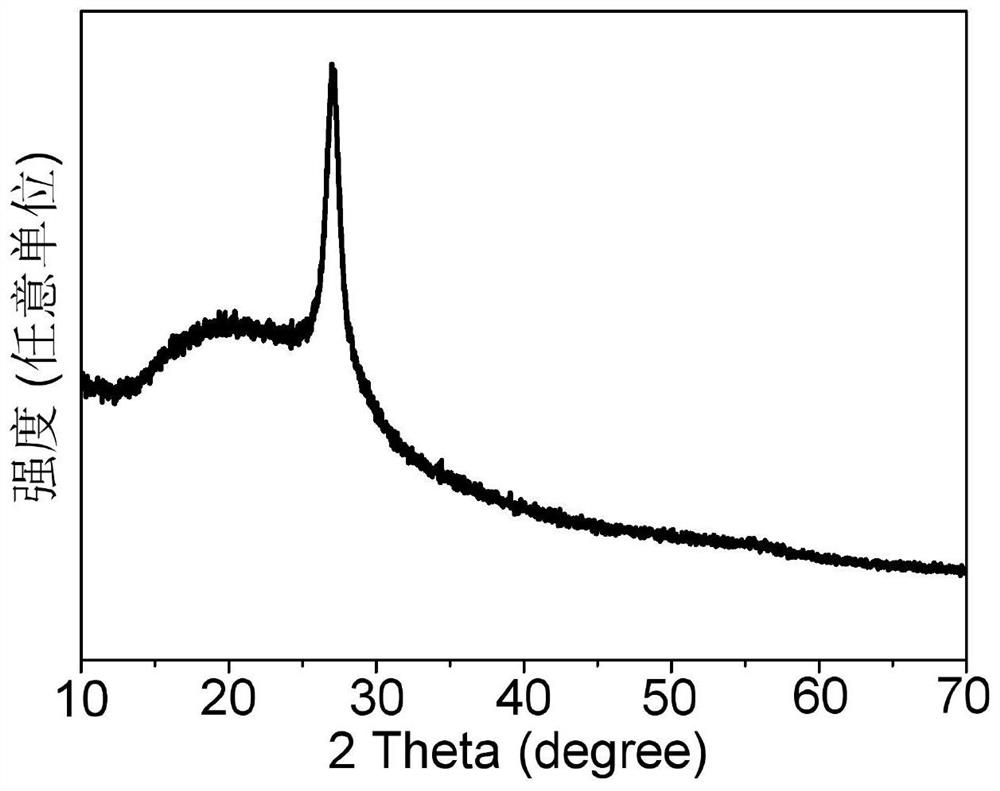
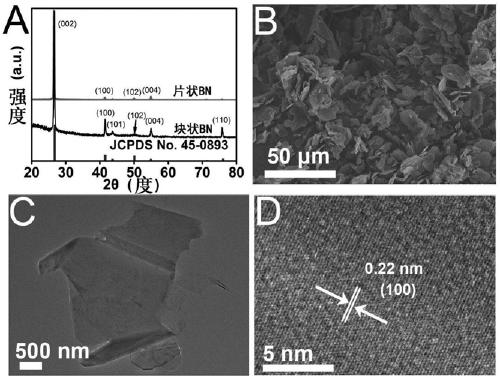
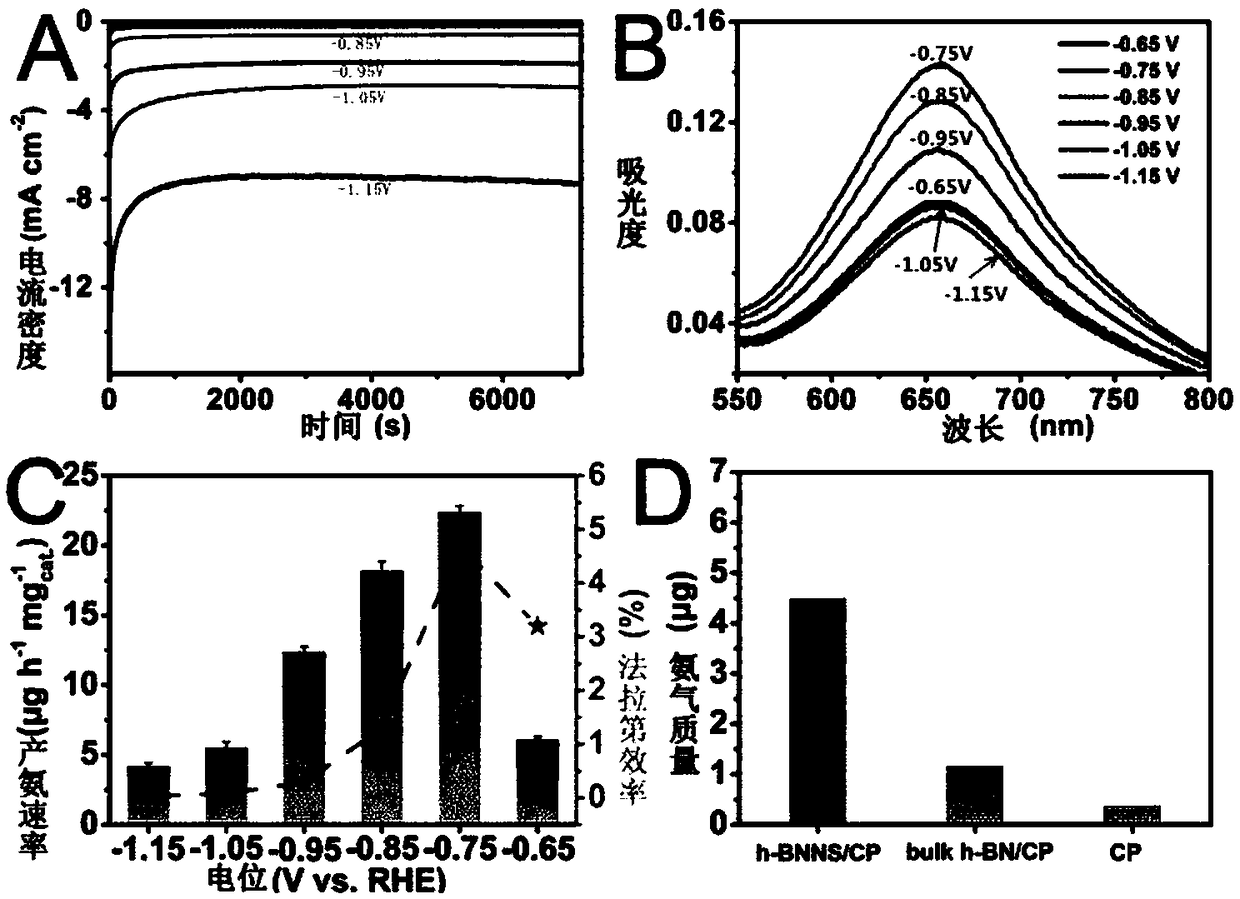
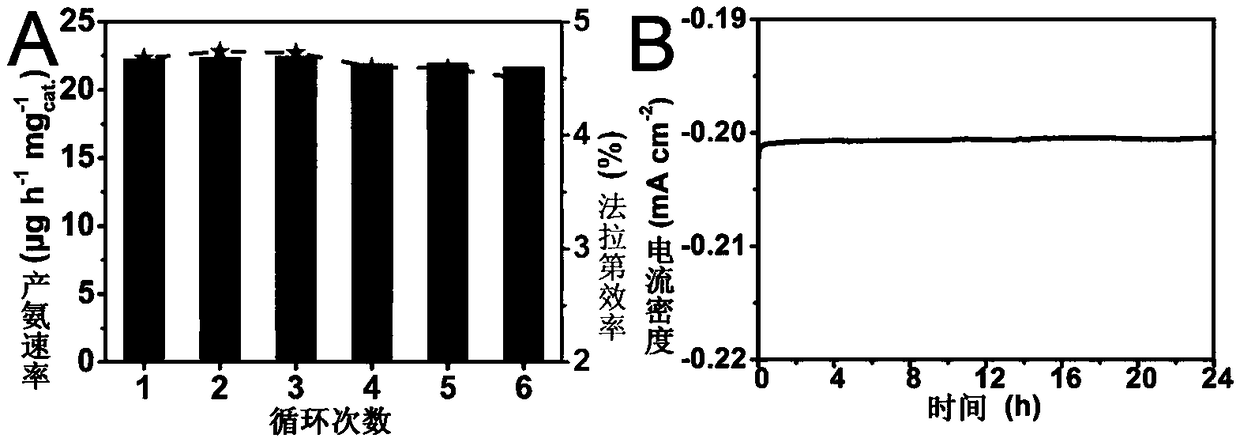
ActiveCN109321930AGood catalytic activity and selectivityThe solution is simpleElectrodesNanometreAmmonia production
The invention relates to application of BN to electrochemical ammonia production, and belongs to the field of nano new materials. A BN nanosheet is used as a nitrogen reduction catalyst to be used forelectrochemical synthesizing of ammonia gas under acidic conditions. According to the application, the BN nanosheet is firstly used as the nitrogen reduction catalyst to be used for electrochemical synthesizing of the ammonia gas under the acidic conditions, high catalytic activity and selectivity are shown, and as a metal-free material, BN is more stable in actual use and is more environment-friendly. According to the application, the treatment scheme of the BN nanosheet is simple and easy, operation is easy, all processes do not involve high temperature reactions, energy is saved, and the application is more consistent with the concept of sustainable development; the BN nanosheet catalyst is used for electrochemical synthesizing of the ammonia gas under the acidic conditions, and the Faraday efficiency as high as 4.7% is shown, and the performance is higher than that of most currently-proven water-based nitrogen reduction catalysts; and in addition, the good selectivity and stability are achieved, and the BN nanosheet is an excellent new nitrogen reduction catalyst.
Owner:QUFU NORMAL UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com