Catalyst for carbon dioxide reduction through electrocatalysis and preparation method thereof
A carbon dioxide and catalyst technology, which is applied in the field of electrocatalytic carbon dioxide reduction catalysts and its preparation, can solve the problems of low selectivity of target products, increased catalyst cost, poor catalyst stability, etc., to improve Faradaic efficiency, increase contact specific surface area, The effect of energy efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
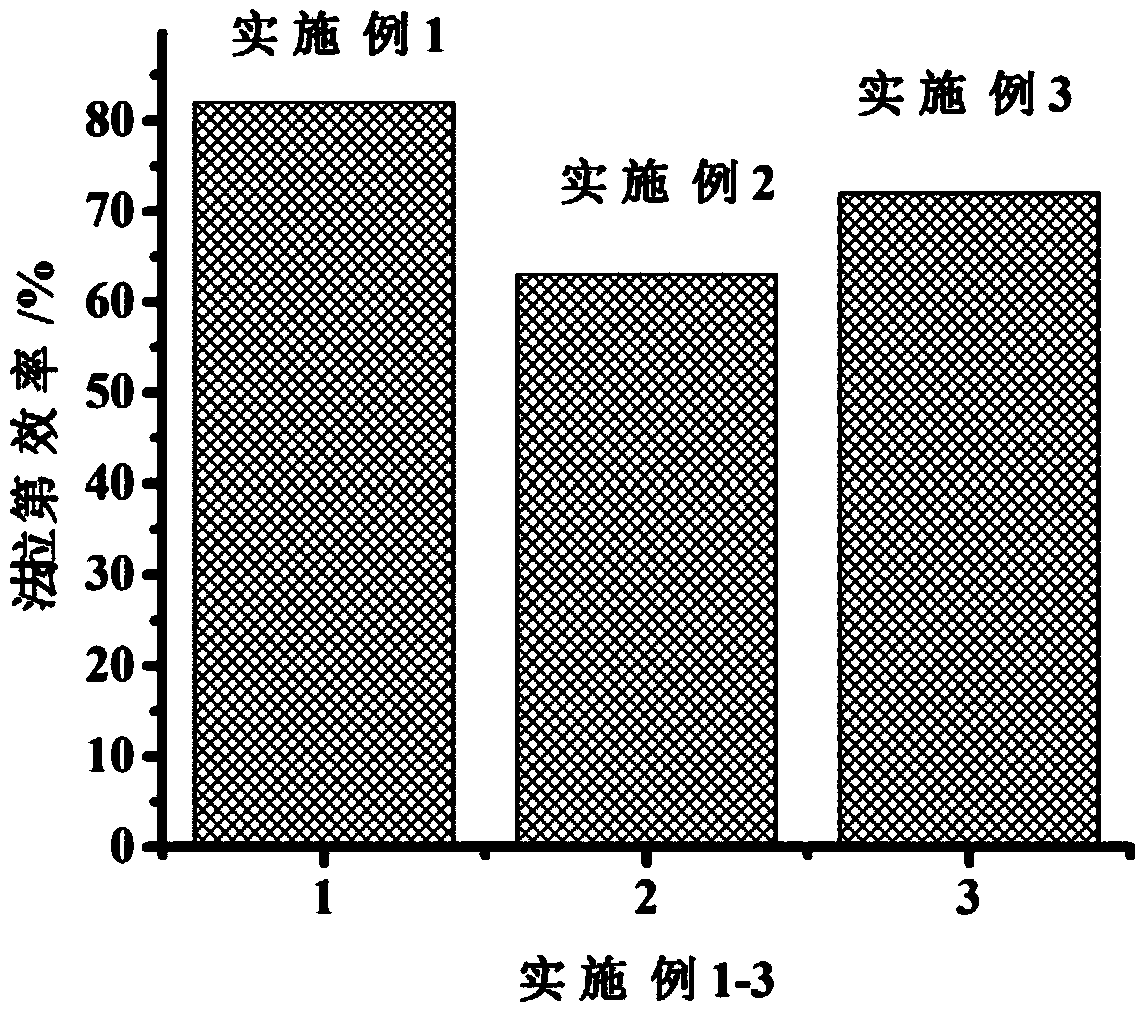
Embodiment 1
[0018] The preparation method of the catalyst for the electrocatalytic reduction of carbon dioxide is characterized in that it comprises the following steps:
[0019] (1) Preparation of MXene: Immerse 1 g of ground MAX phase ceramic powder into 20 mL of HF solution with a mass fraction of 40%, raise the temperature to 80°C, and centrifuge to obtain the product after magnetic stirring for 18 hours, wash with deionized water until neutral , placed in an oven and dried at 70°C for 18 hours to obtain MXene, in which the MAX phase ceramic is Ti 3 AlC 2 、Ti 2 AlC, Cr 2 At least one of AlC; the resulting MXene is Ti 3 C 2 T x (T x -OH, -F and other functional groups), Ti 2 CT x (T x -OH, -F and other functional groups), Cr 2 CT x (T x is at least one of functional groups such as -OH, -F);
[0020] (2) Preparation of indium zinc sulfide-MXene composite material: Take 0.5g of indium nitrate, 1g of thiourea, 1g of zinc nitrate and 0.1g of MXene prepared in step (1) and diss...
Embodiment 2
[0022] The preparation method of the catalyst for the electrocatalytic reduction of carbon dioxide is characterized in that it comprises the following steps:
[0023] (1) Preparation of MXene: Immerse 1 g of ground MAX phase ceramic powder into 20 mL of HF solution with a mass fraction of 30%, raise the temperature to 50°C, and centrifuge to obtain the product after magnetic stirring for 12 hours, wash with deionized water until neutral , placed in an oven and dried at 60°C for 12 hours to obtain MXene, in which the MAX phase ceramic is Ti 3 AlC 2 、Ti 2 AlC, Cr 2 At least one of AlC; the resulting MXene is Ti 3 C 2 T x (T x -OH, -F and other functional groups), Ti 2 CT x (T x -OH, -F and other functional groups), Cr 2 CT x (T x is at least one of functional groups such as -OH, -F);
[0024] (2) Preparation of indium zinc sulfide-MXene composite material: Dissolve 1g of indium nitrate, 2g of thiourea, 2g of zinc nitrate and 0.5g of MXene prepared in step (1) in 100...
Embodiment 3
[0026] The preparation method of the catalyst for the electrocatalytic reduction of carbon dioxide is characterized in that it comprises the following steps:
[0027] (1) Preparation of MXene: Immerse 1 g of ground MAX phase ceramic powder into 20 mL of HF solution with a mass fraction of 50%, raise the temperature to 90°C, and centrifuge to obtain the product after magnetic stirring for 24 hours, wash with deionized water until neutral , placed in an oven and dried at 80°C for 24 hours to obtain MXene, in which the MAX phase ceramic is Ti 3 AlC 2 、Ti 2 AlC, Cr 2 At least one of AlC; the resulting MXene is Ti 3 C 2 T x (T x -OH, -F and other functional groups), Ti 2 CT x (T x -OH, -F and other functional groups), Cr 2 CT x (T x is at least one of functional groups such as -OH, -F);
[0028] (2) Preparation of indium zinc sulfide-MXene composite material: Take 0.8g of indium nitrate, 1.5g of thiourea, 1.5g of zinc nitrate and 0.3g of MXene prepared in step (1) and ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com