Iron-based non-noble metal catalyst for electrocatalytic synthetic ammonia and preparation method thereof
A non-precious metal and catalyst technology, applied in electrodes, electrolysis process, electrolysis components, etc., can solve the problems of low Faradaic efficiency and low catalytic efficiency, and achieve the effects of improving the utilization rate of iron atoms, reducing costs, and convenient preparation methods.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
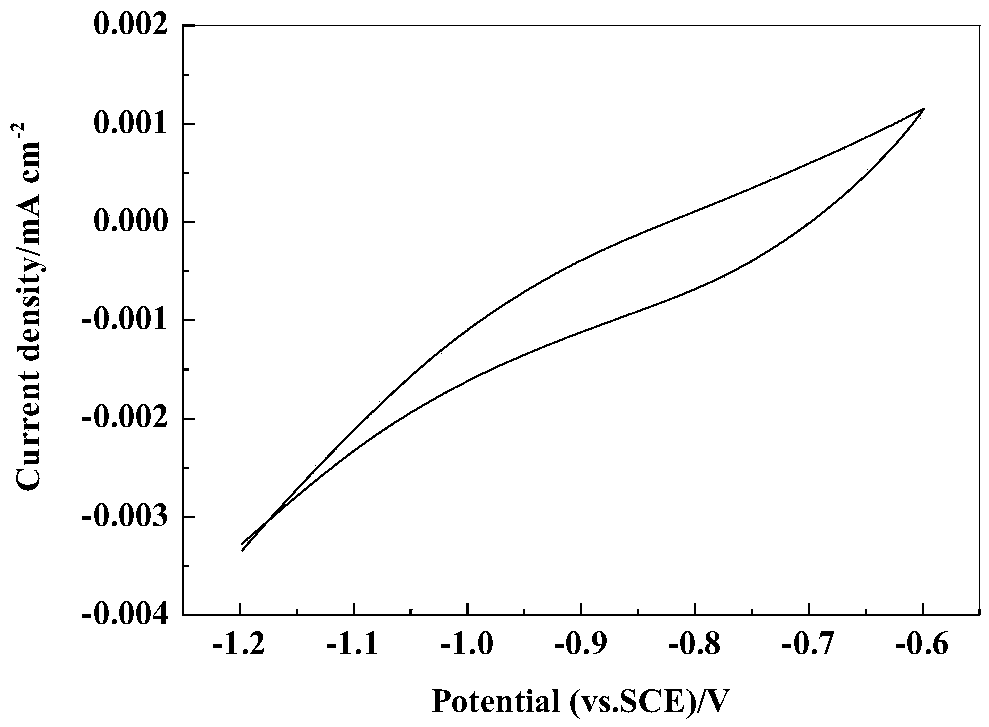
[0041] Weigh FeSO 4 ·7H 2 Add 5.56g of O, 0.1g of ascorbic acid, 0.6g of o-sulfonyl benzimide, and 0.3g of sodium lauryl sulfate into a beaker, add an appropriate amount of deionized water to dissolve, stir until it is completely dissolved, transfer it to a 200mL volumetric flask, and continue Add deionized water to the scale line; before electrodeposition, carry out surface treatment on the graphite rod working electrode to increase surface smoothness or remove surface impurity ions; use platinum electrode as counter electrode, saturated calomel electrode as reference electrode, and graphite rod as working electrode Electrode, take 50mL of FeSO prepared above 4 The solution is placed in a conventional electrolytic cell (such as figure 2 ), adjust the pH to 3.0, use the Gamry electrochemical workstation, use the three-electrode system cyclic voltammetry for electrodeposition, and set the scan rate to 0.02V s -1 , the cycle potential is between -1.6V and -0.8V, and the meta...
Embodiment 2
[0044] Weigh FeSO 4 ·7H 2 Add 8.58g of O, 0.2g of ascorbic acid, 0.8g of o-sulfonyl benzimide, and 0.5g of sodium lauryl sulfate into a beaker, add an appropriate amount of deionized water to dissolve, stir until it is completely dissolved, transfer it to a 200mL volumetric flask, and continue Add deionized water to the scale line; before electrodeposition, carry out surface treatment on the graphite rod working electrode to increase surface smoothness or remove surface impurity ions; use platinum electrode as counter electrode, saturated calomel electrode as reference electrode, and graphite rod as working electrode Electrode, take 50mL of FeSO prepared above 4 The solution was placed in a conventional electrolytic cell, and the pH was adjusted to 3.5. With the help of Gamry electrochemical workstation, three-electrode system cyclic voltammetry was used for electrodeposition, and the scanning rate was set to 0.02V s. -1 , the cycle potential is between -1.6V and -0.8V, and ...
Embodiment 3
[0047] Weigh FeSO 4 ·7H 2 Add 5.56g of O, 0.1g of ascorbic acid, 0.6g of o-sulfonyl benzimide, and 0.3g of sodium lauryl sulfate into a beaker, add an appropriate amount of deionized water to dissolve, stir until it is completely dissolved, transfer it to a 200mL volumetric flask, and continue Add deionized water to the scale line; before electrodeposition, carry out surface treatment on the metal copper sheet working electrode to increase the surface smoothness or remove surface impurity ions; use the platinum electrode as the counter electrode, the saturated calomel electrode as the reference electrode, and the copper sheet as Working electrode, take 50mL of FeSO prepared above 4 The solution was placed in a conventional electrolytic cell, and the pH was adjusted to 3.5. With the help of Gamry electrochemical workstation, three-electrode system cyclic voltammetry was used for electrodeposition, and the scanning rate was set to 0.02V s. -1 , the cycle potential is between -...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com