Preparation method of electrode used for CO2 electrochemical reduction reaction
A carbon dioxide and electrochemical technology, which is applied to battery electrodes, circuits, electrical components, etc., can solve the problems of reducing catalyst life and achieve the effects of increased selectivity, conventional production equipment, and reduced hydrogen evolution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
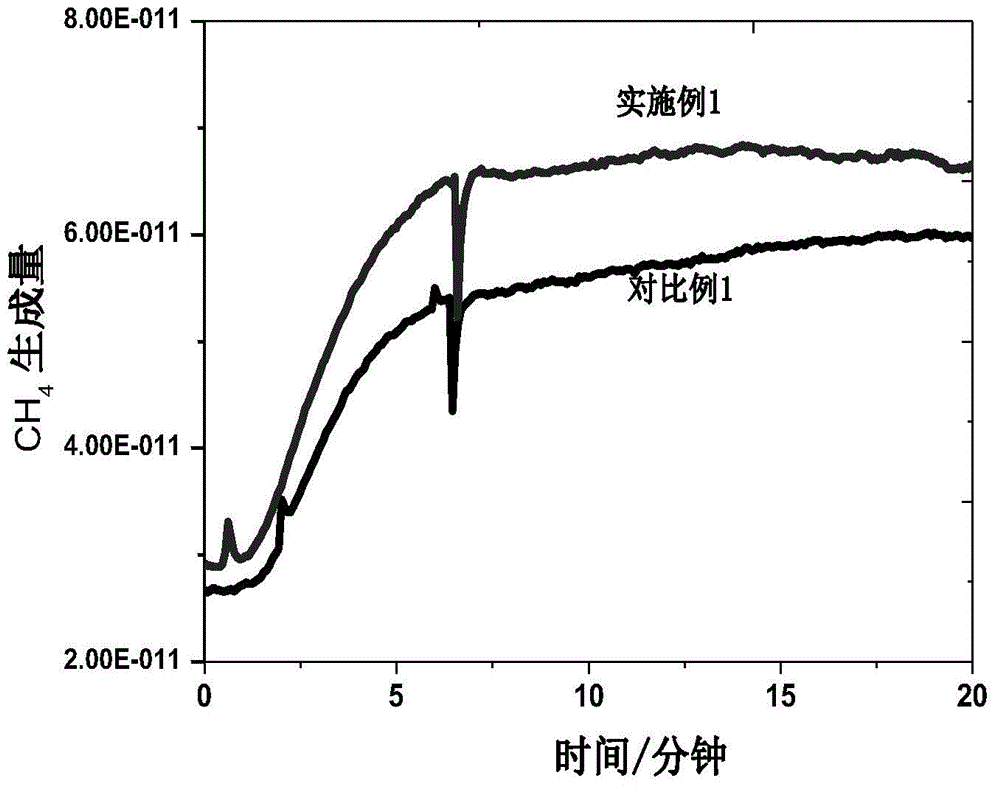
Image
Examples
Embodiment 1
[0026] Treat A in acetone for 30 minutes with copper mesh as the base; treat A in 36.5% concentrated hydrochloric acid for 15 minutes, wash and dry to obtain B; the concentration is 0.2MCuSO 4 Solution, mixed with 0.1M urea at a molar ratio of 1:3 to obtain mixture C; after transferring C to the reactor, immerse B in the solution, seal it, and react at 180°C for 4 hours, then take out the reactor After cooling, pour out the solution, wash, dry, and dry to obtain D; heat-treat D at 500°C for 4 hours under the condition of a nitriding atmosphere to obtain E; put E in a 2M sulfuric acid solution under a nitriding atmosphere, Constant current-100mA / cm 2 Scan for 10 minutes, wash and dry to obtain the electrode.
Embodiment 2
[0028]Treat A in acetone for 30 minutes with copper mesh as the base; treat A in 36.5% concentrated hydrochloric acid for 15 minutes, wash and dry to obtain B; mix 0.5M copper acetate solution with 0.2M template agent CTAB, Mix evenly at a molar ratio of 1:2 to obtain mixture C; transfer C to the reactor, immerse B in the solution, seal it, react at 160°C for 4 hours, take out the reactor and cool it, and pour out the solution , washed, dried, and dried to obtain D; heat-treated D at 500°C for 4 hours under the condition of a nitriding atmosphere to obtain E; put E in a 0.5M sulfuric acid solution under a nitriding atmosphere at a constant current of -20mA / cm 2 Scan for 20min, wash and dry to get the electrode.
Embodiment 3
[0030] Treat A in acetone for 30 minutes with copper mesh as the base; treat A in 36.5% concentrated hydrochloric acid for 15 minutes, wash and dry to obtain B; mix 0.5M copper acetate solution with 0.2M template agent CTAB, Mix evenly at a molar ratio of 1:2 to obtain mixture C; transfer C into the reactor, immerse B in the solution, seal it, react at 150°C for 4 hours, take out the reactor and cool it, and pour out the solution , washed, dried, and dried to obtain D; D was heat-treated at 500°C for 4 hours under the condition of a nitriding atmosphere to obtain E; E was placed in a 0.5M sulfuric acid solution under a nitriding atmosphere, and the constant current was -40mA / cm 2 Scan for 20min, wash and dry to get the electrode.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com