Method for preparing furan-2,5-dicarboxylicacid from 5-hydroxymethylfurfural through electrochemical oxidation
A technology of furandicarboxylic acid and hydroxymethylfurfural, which is applied in chemical instruments and methods, physical/chemical process catalysts, chemical/physical processes, etc., can solve the problems of low current density, reaction selectivity and efficiency limitation, and achieve current High efficiency, reduced oxygen evolution reaction and catalyst deactivation effect, and improved current efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] Example 1: Preparation of reduced catalyst and working electrode
[0057] Weigh 0.2000g of activated carbon, 0.2310g of copper nitrate trihydrate and 0.0930g of nickel nitrate hexahydrate, add them into 200mL of ultrapure water and mix, called solution A, the stirring rate is 1500r / min, and the stirring time is 30min. Weigh 3.3800g of sodium borohydride, dissolve it in 50mL of solution, called solution B, slowly drop solution B into solution A, keep stirring, and the reaction time is 4h. After filtration, it was washed three times with ultrapure water, and the filter cake was transferred to a vacuum drying oven. The drying time was 12 hours, and the obtained solid was ground to obtain a reduced state copper-nickel catalyst supported by activated carbon, which was 5-hydroxymethylfurfural electrocatalyst. The chemical catalyst is called 3CuNi@C (40wt%) catalyst.
[0058] Disperse 20 mg of the synthesized activated carbon-supported reduced copper-nickel catalyst into 1000...
Embodiment 2
[0059] Embodiment 2: Preparation of reduced state catalyst and working electrode
[0060] Weigh 0.2000g of activated carbon, 0.2070g of copper nitrate trihydrate and 0.1246g of nickel nitrate hexahydrate, add them into 200mL of ultrapure water and mix them, called solution A, the stirring rate is 1500r / min, and the stirring time is 30min. Weigh 3.4041g of sodium borohydride, dissolve it in 50mL of solution, called solution B, slowly add solution B to solution A, keep stirring, and the reaction time is 4h. After filtration, it was washed three times with ultrapure water, and the filter cake was transferred to a vacuum drying oven. The drying time was 12 hours, and the obtained solid was ground to obtain a reduced state copper-nickel catalyst supported by activated carbon, which was 5-hydroxymethylfurfural electrocatalyst. The chemical catalyst is called 2CuNi@C (40wt%) catalyst.
[0061] The working electrode was prepared according to the method shown in Example 1.
Embodiment 3
[0062] Embodiment 3: Preparation of reduced state catalyst and working electrode
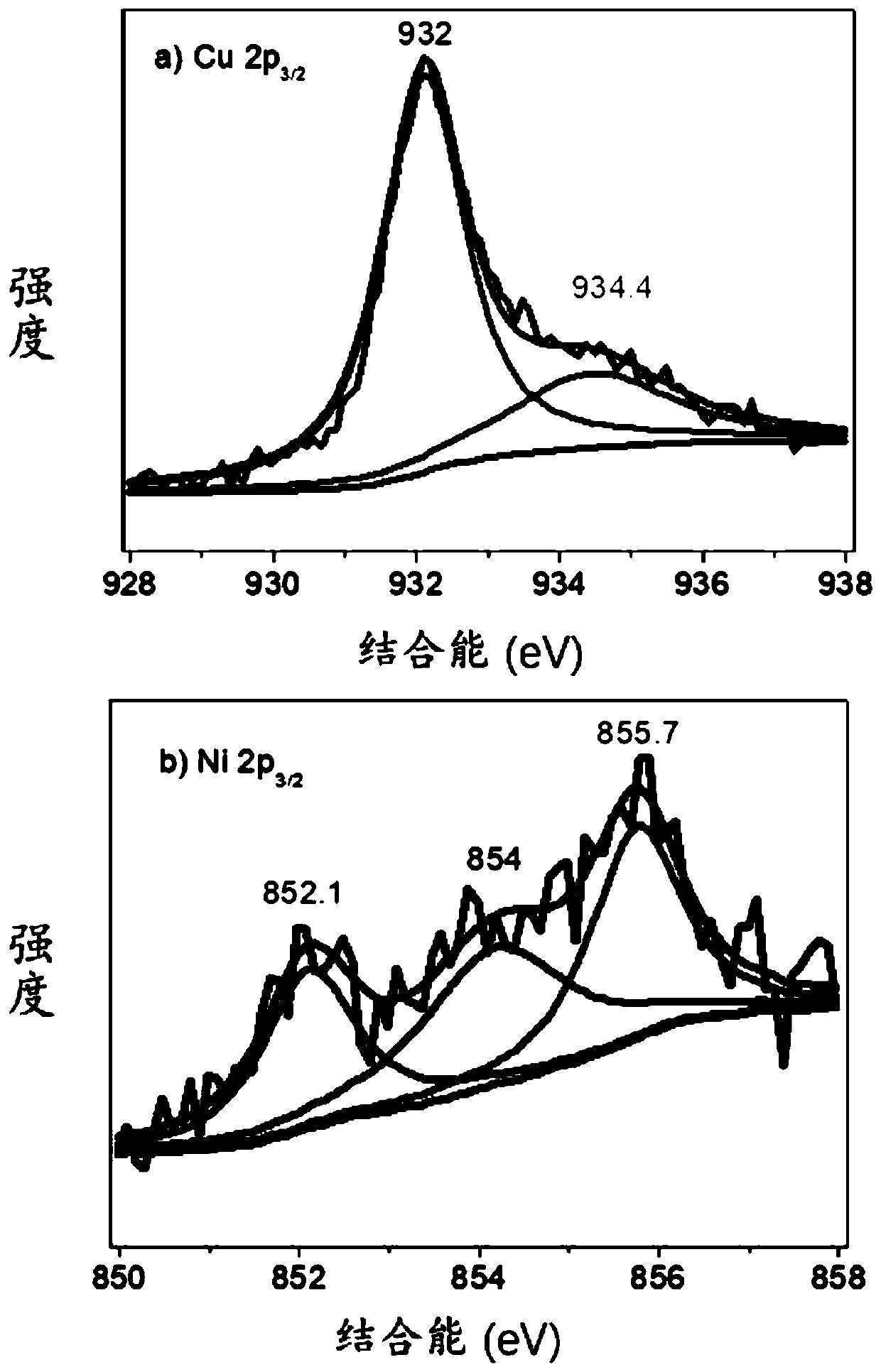
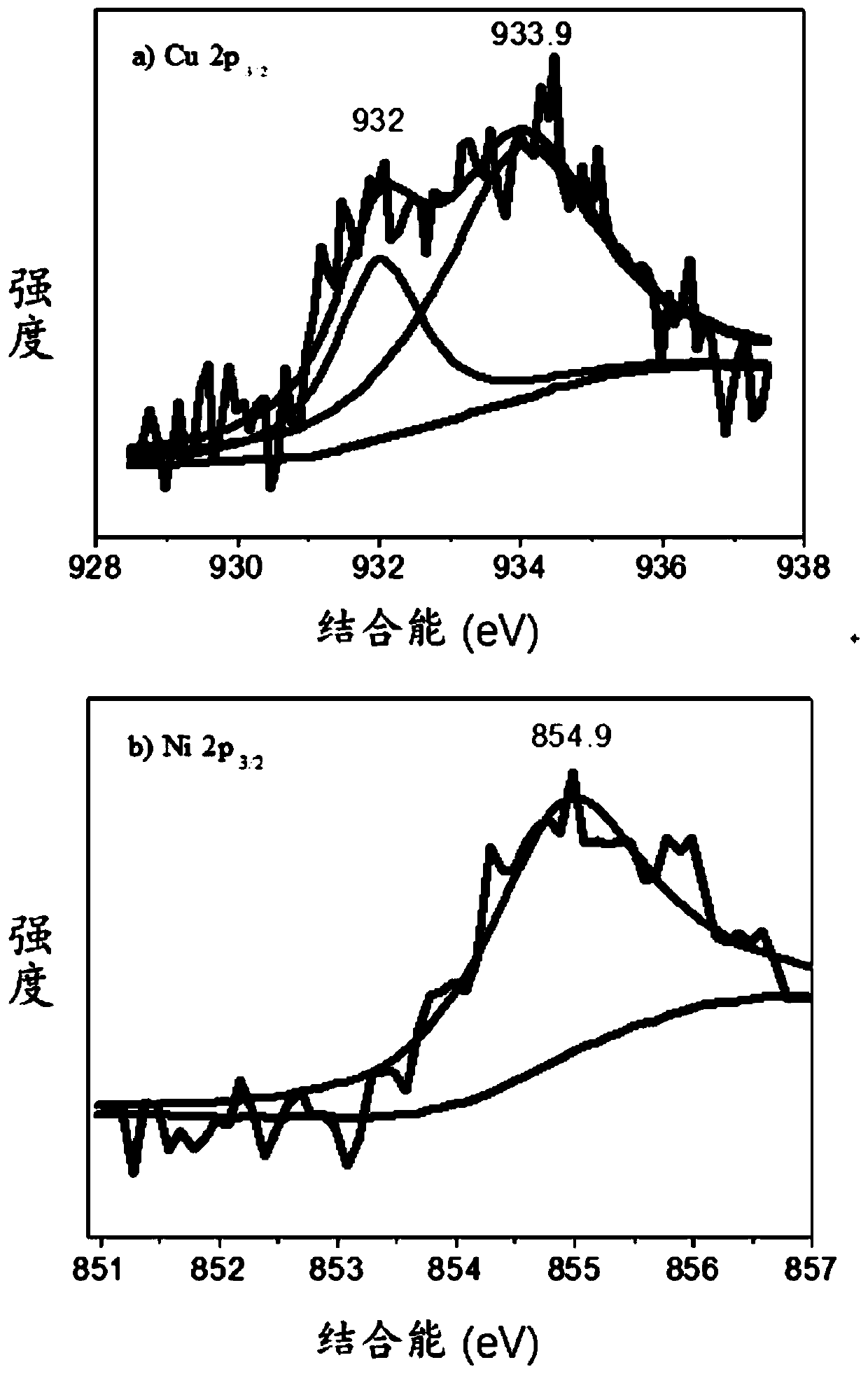
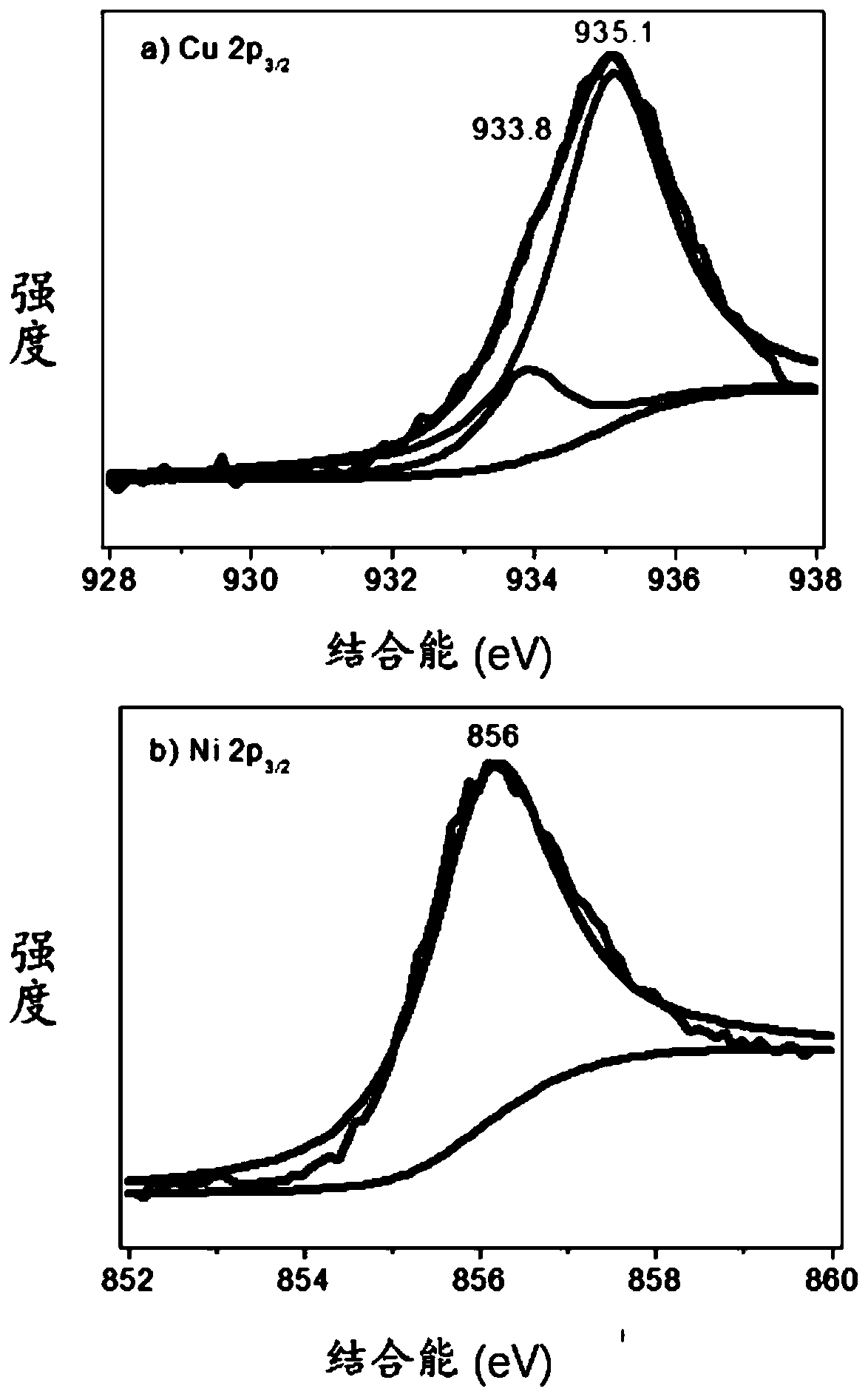
[0063] Weigh 0.2000g of activated carbon, 0.1575g of copper nitrate trihydrate and 0.1896g of nickel nitrate hexahydrate, add them into 200mL of ultrapure water and mix them, called solution A, the stirring rate is 1500r / min, and the stirring time is 30min. Weigh 3.4531g of sodium borohydride, dissolve it in 50mL of solution, called solution B, slowly drop solution B into solution A, keep stirring, and the reaction time is 4h. After filtration, it was washed three times with ultrapure water, and the filter cake was transferred to a vacuum drying oven. The drying time was 12 hours, and the obtained solid was ground to obtain a reduced state copper-nickel catalyst supported by activated carbon, which was 5-hydroxymethylfurfural electrocatalyst. The chemical catalyst is called CuNi@C (40wt%) catalyst. The XPS curve figure of the CuNi@C (40wt%) catalyst prepared in this embodiment is as follows fi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com