Ni-N-C catalyst for electroreduction reaction of carbon dioxide and preparation and application thereof
A carbon dioxide and catalyst technology, applied in electrodes, electrolytic processes, electrolytic components, etc., can solve the problems of insufficient carbon dioxide electroreduction activity and increased current density of CO, and achieve excellent carbon dioxide electroreduction activity and high current density.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
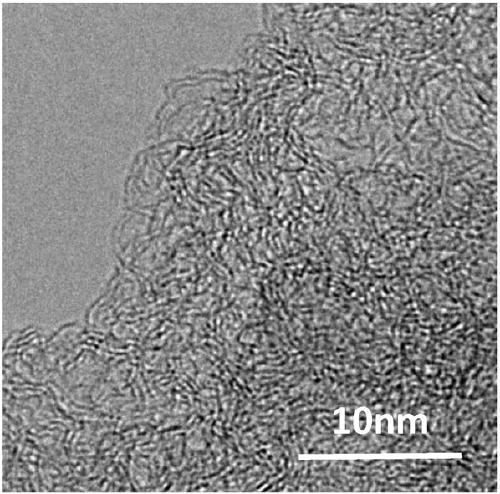
[0046] Weigh 2.45g Zn(NO 3 ) 2 ·6H 2 O and 2.39g Ni(NO 3 ) 2 ·6H 2 O was dissolved in 300mL of methanol, and 300mL of methanol solution containing 10.82g of 2-dimethylimidazole was added under stirring, and the stirring was continued at room temperature for 12 hours; centrifuged and washed with methanol for 3 times, and dried in vacuum to obtain a solid powder; take 0.2g of the solid powder Placed in a quartz boat, placed in a tube furnace, 50mLmin -1 Under argon protection, 5°C min -1 Heating to 900°C and maintaining it for 4 hours, cooling to room temperature to obtain a Ni-N-C catalyst. like figure 1 As shown, the Ni-N-C catalyst was in the form of a porous carbon layer, and no metal particles were observed.
[0047] Weigh 4.4 mg of Ni-N-C catalyst, add appropriate amount of deionized water and ethanol, mix ultrasonically for 10 minutes, add 9.8 mg of 5% Nafion solution, and mix ultrasonically for 20 minutes to obtain catalyst slurry. The catalyst slurry was brushe...
Embodiment 2
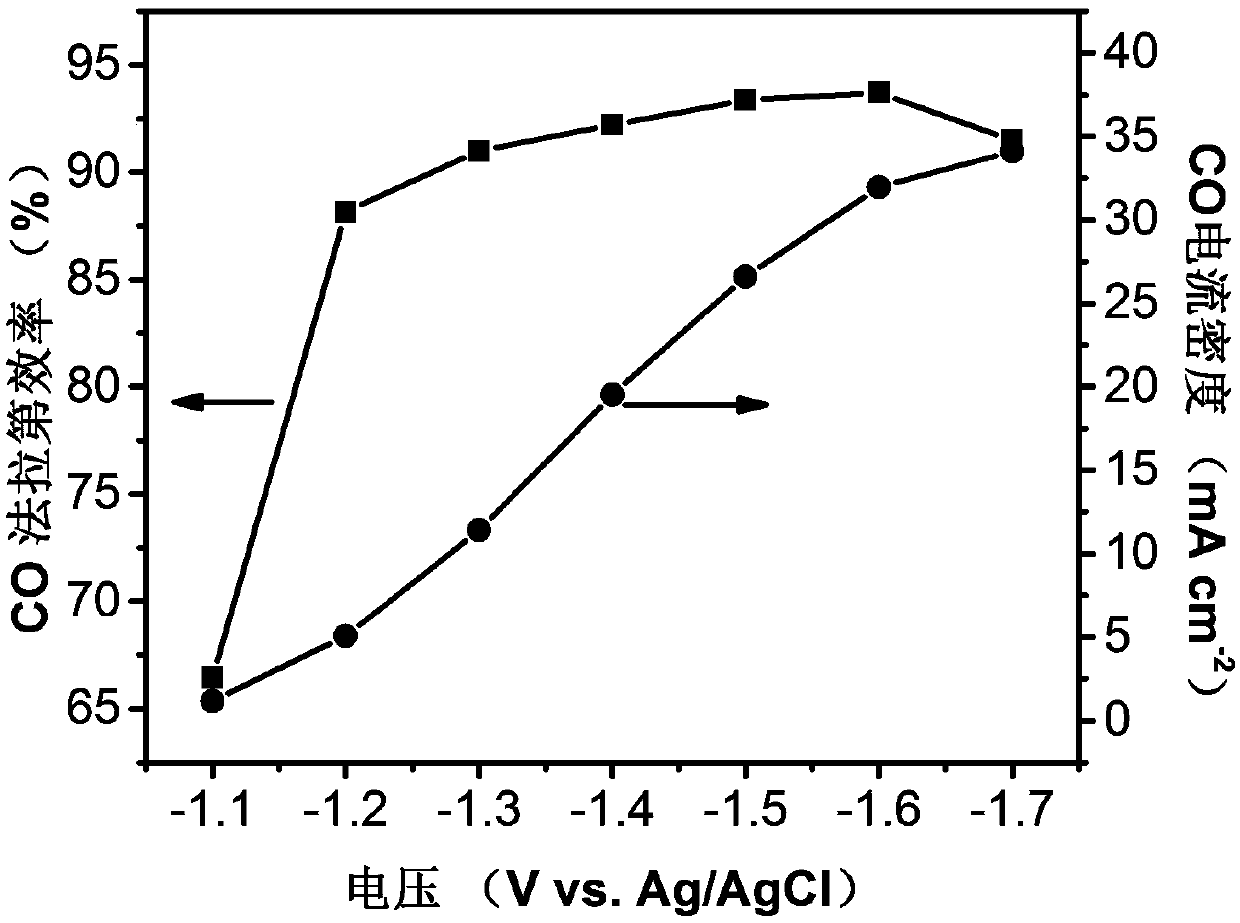
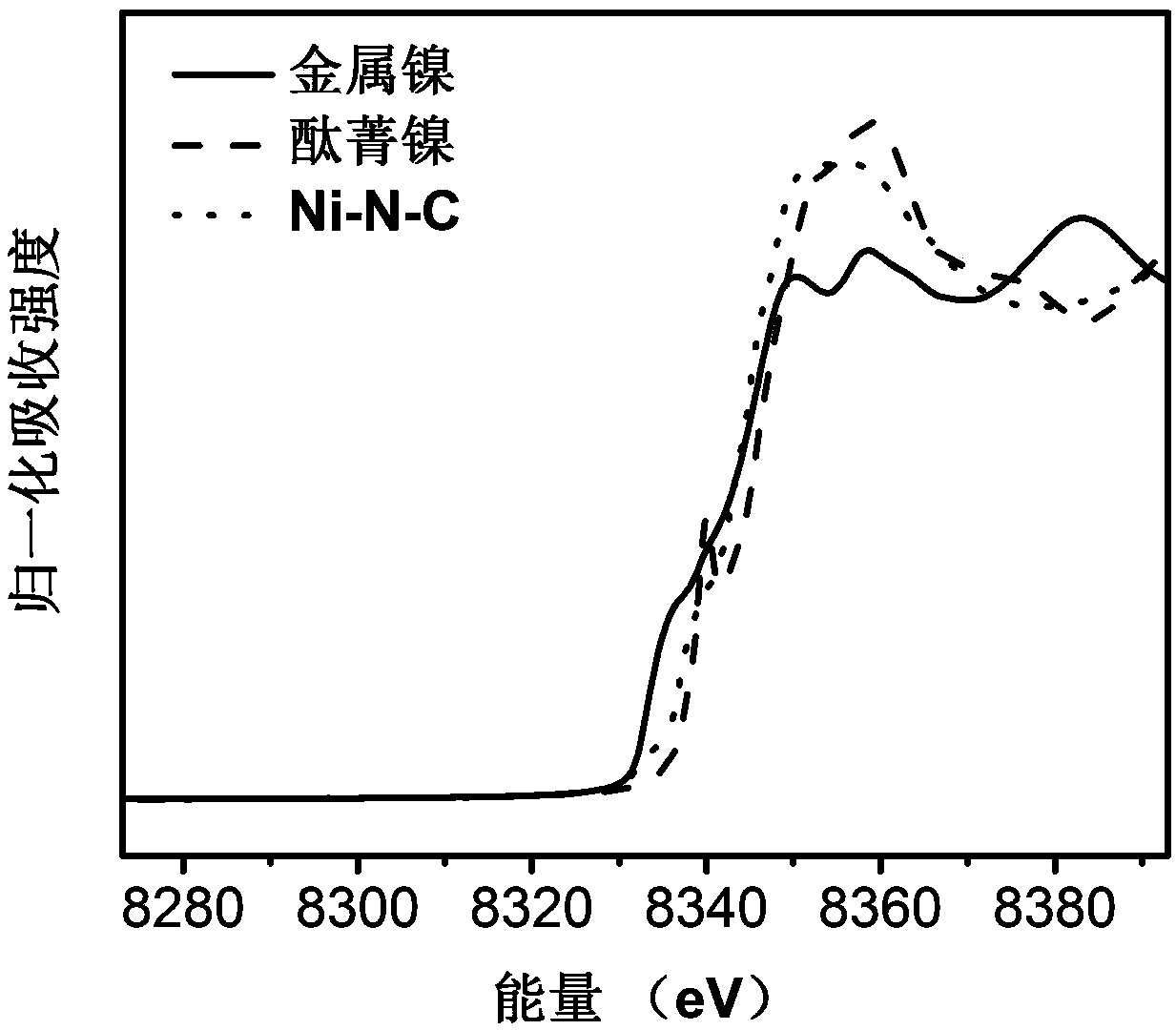
[0051] Weigh 2.94g Zn(NO 3 ) 2 ·6H 2 O and 11.47g Ni(NO 3 ) 2 ·6H 2 O was dissolved in 300mL of methanol, and 300mL of methanol solution containing 32.45g of 2-dimethylimidazole was added under stirring, and the stirring was continued at room temperature for 24 hours; centrifuged and washed 3 times with methanol, and dried in vacuo to obtain a solid powder; 0.4g of the solid powder was obtained Placed in a quartz boat, placed in a tube furnace, 100mLmin -1 Under nitrogen protection, 10°C min -1 Heating to 600°C and maintaining it for 12 hours, cooling to room temperature to obtain a Ni-N-C catalyst. like image 3 As shown, the near-edge absorption peak profile of Ni-N-C is between that of simple nickel and nickel phthalocyanine, which proves that the valence state of Ni is between +2 and 0. like Figure 4 As shown, the Ni-N-C catalyst obtained by this method can also electroreduce carbon dioxide to carbon monoxide, but the highest CO Faradaic efficiency is only 65%, a...
Embodiment 3
[0053] Weigh 2.94g Zn(NO 3 ) 2 ·6H 2 O and 1.43g Ni(NO 3 ) 2 ·6H 2 O was dissolved in 300mL of methanol, and 300mL of methanol solution containing 4.86g of 2-dimethylimidazole was added under stirring, and the stirring was continued at room temperature for 24 hours; centrifuged and washed 3 times with methanol, and dried in vacuo to obtain a solid powder; 0.6g of the solid powder was obtained Placed in a quartz boat, placed in a tube furnace, 80mL min -1 Under nitrogen protection, 1°C min -1 Heating to 1000°C and maintaining it for 4 hours, cooling to room temperature to obtain Ni-N-C catalyst.
[0054] The working electrode preparation process and test conditions are the same as in Example 1. like Figure 5 As shown, the Ni-N-C catalyst is in the potential range of -1.2V to -1.6V vs.Ag / AgCl, the CO faradaic efficiency is maintained above 91%, and the highest can reach 95.6%, and the CO sub-current density is -1.7V vs.Ag / AgCl up to 45.6mA cm -2 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com